A randomized feasibility trial of clonidine to reduce perioperative cardiac risk in patients on chronic beta-blockade: the EPIC study
- PMID: 25189430
- PMCID: PMC4194011
- DOI: 10.1007/s12630-014-0226-6
A randomized feasibility trial of clonidine to reduce perioperative cardiac risk in patients on chronic beta-blockade: the EPIC study
Abstract
Purpose: Clonidine may help prevent cardiac complications in patients undergoing non-cardiac surgery and receiving chronic beta-blocker therapy. We conducted a multicentre pilot randomized trial to estimate recruitment rates for a full-scale trial and to assess the safety and tolerability of combining clonidine with chronic beta-blockade.
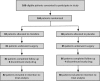
Methods: Patients who were at elevated perioperative cardiac risk, receiving chronic beta-blockade, and scheduled for major non-cardiac surgery were recruited in a blinded (participants, clinicians, outcome assessors) placebo-controlled randomized trial at three Canadian hospitals. Participants were randomized to clonidine (0.2 mg oral tablet one hour before surgery, plus 0.2 mg·day(-1) transdermal patch placed one hour before surgery and removed four days after surgery or hospital discharge, whichever came first) or matching placebo. Feasibility was evaluated based on recruitment rates, with each centre being required to recruit 50 participants within 12-18 months. Additionally, we reviewed study drug withdrawals and safety outcomes, including clinically significant hypotension or bradycardia.
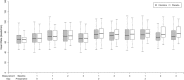
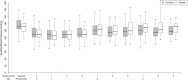
Results: Eighty-two of the 168 participants were randomized to receive clonidine and 86 to receive placebo. The average time to recruit 50 participants at each centre was 14.3 months. Six patients (7%) withdrew from clonidine, while four (5%) withdrew from placebo. Based on qualitative review, there were no major safety concerns related to clonidine. There was a moderate overall rate of cardiac morbidity, with 18 participants (11%) suffering postoperative myocardial infarction.
Conclusion: This pilot randomized trial confirmed the feasibility, safety, and tolerability of a full-scale trial of oral and transdermal clonidine for reducing the risk of cardiac complications during non-cardiac surgery. This trial was registered at www.clinicaltrials.gov: NCT00335582.
Objectif: La clonidine pourrait contribuer à prévenir les complications cardiaques chez des patients subissant une chirurgie non cardiaque et recevant un traitement chronique par bêta-bloqueurs. Nous avons dirigé une étude pilote randomisée, multicentrique, pour estimer les vitesses de recrutement d’une étude définitive et pour évaluer l’innocuité et la tolérabilité d’une association de la clonidine avec des bêta-bloqueurs administrés en traitement chronique.
Méthodes: Des patients qui présentaient un risque cardiaque périopératoire élevé, recevaient un traitement chronique par bêta-bloqueurs et devaient subir une intervention chirurgicale majeure non cardiaque programmée ont été recrutés dans une étude randomisée en insu (participants, cliniciens, évaluateurs de l’aboutissement), contrôlée par placebo, dans trois hôpitaux canadiens. Les participants ont été randomisés pour recevoir de la clonidine (un comprimé de 0,2 mg per os une heure avant l’opération et 0,2 mg·jour−1 en timbre épidermique placé une heure avant l’opération et retiré quatre jours plus tard ou au moment du congé de l’hôpital, selon ce qui survenait en premier) ou un placebo correspondant. La faisabilité a été évaluée en fonction des vitesses de recrutement, chaque centre devant recruter 50 participants dans un délai de 12 à 18 mois. De plus, nous avons analysé les arrêts prématurés du traitement étudié et les résultats d’innocuité, y compris les épisodes cliniquement significatifs d’hypotension ou de bradycardie.
Résultats: Quatre-vingt-deux des 168 participants ont été randomisés dans le groupe recevant la clonidine et 86 dans le groupe recevant le placebo. Le délai moyen de recrutement des 50 participants dans chaque centre a été de 14,3 mois. Six patients (7 %) ont été retirés du groupe clonidine et quatre patients (5 %) ont été retirés du groupe placebo. Sur le plan qualitatif, l’analyse n’a relevé aucun problème majeur d’innocuité lié à la clonidine. Il y a eu un taux global modéré de morbidité cardiaque, 18 participants (11 %) souffrant d’infarctus du myocarde postopératoire.
Conclusion: Cette étude pilote randomisée a confirmé la faisabilité, l’innocuité et la tolérabilité d’une étude à grande échelle de l’administration orale et transdermique de la clonidine sur la réduction du risque de complications cardiaques au cours d’une chirurgie non cardiaque. Cette étude a été enregistrée sur le site www.clinicaltrials.gov: NCT00335582.
Figures
References
-
- The Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307: 2295-304. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

