MYC activates stem-like cell potential in hepatocarcinoma by a p53-dependent mechanism
- PMID: 25189530
- PMCID: PMC4199878
- DOI: 10.1158/0008-5472.CAN-14-0527
MYC activates stem-like cell potential in hepatocarcinoma by a p53-dependent mechanism
Abstract
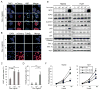
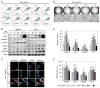
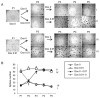
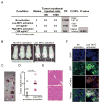
Activation of c-MYC is an oncogenic hallmark of many cancers, including liver cancer, and is associated with a variety of adverse prognostic characteristics. Despite a causative role during malignant transformation and progression in hepatocarcinogenesis, consequences of c-MYC activation for the biology of hepatic cancer stem cells (CSC) are undefined. Here, distinct levels of c-MYC overexpression were established by using two dose-dependent tetracycline-inducible systems in four hepatoma cell lines with different p53 mutational status. The CSCs were evaluated using side population (SP) approach as well as standard in vitro and in vivo assays. Functional repression of p53 was achieved by lentiviral shRNA transduction. The results show that c-MYC expression levels have a differential impact on liver CSC characteristics. At low levels, c-MYC activation led to increased proliferation and enhanced CSC properties including activation of reprogramming transcription factors and CSC marker expression (e.g., NANOG, OCT4, and EpCAM), expansion of SP, and acceleration of tumor growth upon subcutaneous transplantation into immunocompromised mice. However, when exceeding a threshold level, c-MYC induced a proapoptotic program and loss of CSC potential both in vitro and in vivo. Mechanistically, c-MYC-induced self-renewal capacity of liver cancer cells was exerted in a p53-dependent manner. Low c-MYC activation increased spheroid formation in p53-deficient tumor cells, whereas p53-dependent effects were blunted in the absence of c-MYC overexpression. Together, our results confirm the role of c-MYC as a master regulator during hepatocarcinogenesis and establish a new gatekeeper role for p53 in repressing c-MYC-induced CSC phenotype in liver cancer cells.
©2014 American Association for Cancer Research.
Conflict of interest statement
Figures



References
-
- Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

