Impact and cost-effectiveness of current and future tuberculosis diagnostics: the contribution of modelling
- PMID: 25189546
- PMCID: PMC4436823
- DOI: 10.5588/ijtld.13.0851
Impact and cost-effectiveness of current and future tuberculosis diagnostics: the contribution of modelling
Abstract
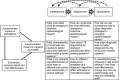
The landscape of diagnostic testing for tuberculosis (TB) is changing rapidly, and stakeholders need urgent guidance on how to develop, deploy and optimize TB diagnostics in a way that maximizes impact and makes best use of available resources. When decisions must be made with only incomplete or preliminary data available, modelling is a useful tool for providing such guidance. Following a meeting of modelers and other key stakeholders organized by the TB Modelling and Analysis Consortium, we propose a conceptual framework for positioning models of TB diagnostics. We use that framework to describe modelling priorities in four key areas: Xpert(®) MTB/RIF scale-up, target product profiles for novel assays, drug susceptibility testing to support new drug regimens, and the improvement of future TB diagnostic models. If we are to maximize the impact and cost-effectiveness of TB diagnostics, these modelling priorities should figure prominently as targets for future research.
Le paysage des tests de laboratoire de la tuberculose (TB) change rapidement et les parties prenantes ont besoin de directives urgentes sur la manière d'élaborer, de diffuser et d'optimiser le diagnostic de la TB de façon à maximiser son impact et faire le meilleur usage des ressources disponibles. Quand il faut prendre des décisions basées sur les données incomplètes ou préliminaires qui sont les seules disponibles, la modélisation est un outil utile pour fournir ce type de directive. A la suite d'une réunion de modélisateurs et d'autres intervenants majeurs organisée par le « TB Modelling and Analysis Consortium', nous proposons un cadre conceptuel pour intégrer de nouveaux modèles de diagnostic de la TB. Nous utilisons ce cadre pour décrire les priorités de la modélisation dans quatre domaines principaux : l'expansion du test Xpert® MTB/RIF, le ciblage de produits pour de nouveaux tests, les tests de pharmaco-sensibilité afin d'élaborer de nouveaux protocoles thérapeutiques et la nécessité d'améliorer les modèles futurs de diagnostic de la TB. Si nous voulons maximiser l'impact et la rentabilité du diagnostic de la TB, ces priorités doivent être les cibles principales de la recherche future.
El panorama de las pruebas diagnósticas de la tuberculosis (TB) está evolucionando rápidamente y los interesados directos precisan con urgencia directrices en materia de desarrollo, despliegue y optimización de estos métodos, de manera que se obtenga el máximo impacto, mediante el uso óptimo de los recursos existentes. Cuando se deben adoptar decisiones solo con base en datos incompletos o preliminares, la modelización aparece como un instrumento útil que puede aportar esta orientación. Tras una reunión con modeladores y otros interesados clave, organizada por el ‘TB Modelling and Analysis Consortium’, se propuso un marco teórico destinado al posicionamiento de los modelos de pruebas diagnósticas de la TB. A partir de este marco, se describen las prioridades de la modelización en cuatro esferas principales: las estrategias de ampliación de escala de la prueba Xpert® MTB/RIF, la definición de las características de las nuevas pruebas, las pruebas de sensibilidad a los medicamentos que fundamenten los nuevos regímenes terapéuticos y las deficiencias que se deben superar a fin de mejorar los futuros modelos de métodos diagnósticos de la TB. Si se busca obtener el máximo impacto y la mayor rentabilidad de los medios diagnósticos, estas prioridades de modelización deben ocupar un puesto prominente en los objetivos de las investigaciones futuras.
Figures
References
-
- Grosset J H, Singer T G, Bishai W R. New drugs for the treatment of tuberculosis: hope and reality. Int J Tuberc Lung Dis. 2012;16:1005–1014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

