Immune checkpoint inhibitors in clinical trials
- PMID: 25189716
- PMCID: PMC4190433
- DOI: 10.5732/cjc.014.10122
Immune checkpoint inhibitors in clinical trials
Abstract
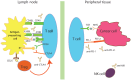
Immunology-based therapy is rapidly developing into an effective treatment option for a surprising range of cancers. We have learned over the last decade that powerful immunologic effector cells may be blocked by inhibitory regulatory pathways controlled by specific molecules often called "immune checkpoints." These checkpoints serve to control or turn off the immune response when it is no longer needed to prevent tissue injury and autoimmunity. Cancer cells have learned or evolved to use these mechanisms to evade immune control and elimination. The development of a new therapeutic class of drugs that inhibit these inhibitory pathways has recently emerged as a potent strategy in oncology. Three sets of agents have emerged in clinical trials exploiting this strategy. These agents are antibody-based therapies targeting cytotoxic T-lymphocyte antigen 4 (CTLA4), programmed cell death 1 (PD-1), and programmed cell death ligand 1 (PD-L1). These inhibitors of immune inhibition have demonstrated extensive activity as single agents and in combinations. Clinical responses have been seen in melanoma, renal cell carcinoma, non-small cell lung cancer, and several other tumor types. Despite the autoimmune or inflammatory immune-mediated adverse effects which have been seen, the responses and overall survival benefits exhibited thus far warrant further clinical development.
Figures
References
-
- Hotchkiss RS, Moldawer LL. Parallels between cancer and infectious disease. N Engl J Med. 2014;371:380–383. - PubMed
-
- Hamanishi J, Mandai M, Ikeda T, et al. Efficacy and safety of anti-PD-1 antibody (Nivolumab: BMS-936558, ONO-4538) in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2014;(32 suppl):abstr 5511.
-
- Seiwert TY, Burtness B, Weiss J, et al. A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV-associated head and neck (H/N) cancer. J Clin Oncol. 2014;(32 suppl):abstr 6011.
-
- Powles T, Vogelzang NJ, Fine GD, et al. Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC) J Clin Oncol. 2014;(32 suppl):abstr 5011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
