Mesalazine in the initial management of severely acutely malnourished children with environmental enteric dysfunction: a pilot randomized controlled trial
- PMID: 25189855
- PMCID: PMC4243388
- DOI: 10.1186/s12916-014-0133-2
Mesalazine in the initial management of severely acutely malnourished children with environmental enteric dysfunction: a pilot randomized controlled trial
Abstract
Background: Environmental enteric dysfunction (EED) is an acquired syndrome of impaired gastrointestinal mucosal barrier function that is thought to play a key role in the pathogenesis of stunting in early life. It has been conceptualized as an adaptive response to excess environmental pathogen exposure. However, it is clinically similar to other inflammatory enteropathies, which result from both host and environmental triggers, and for which immunomodulation is a cornerstone of therapy.
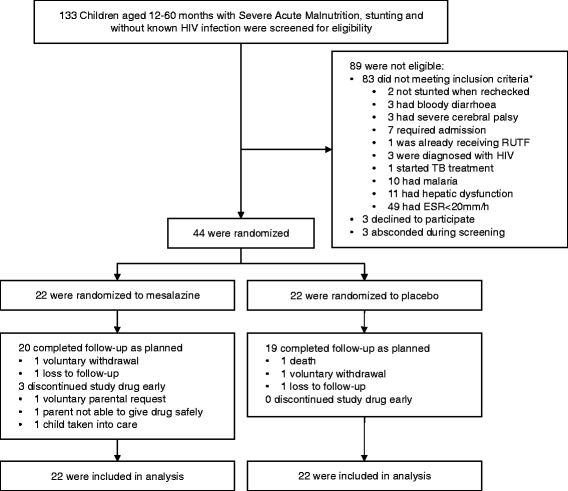
Methods: In this pilot double-blind randomized placebo-controlled trial, 44 children with severe acute malnutrition and evidence of EED were assigned to treatment with mesalazine or placebo for 28 days during nutritional rehabilitation. Primary outcomes were safety and acceptability of the intervention.
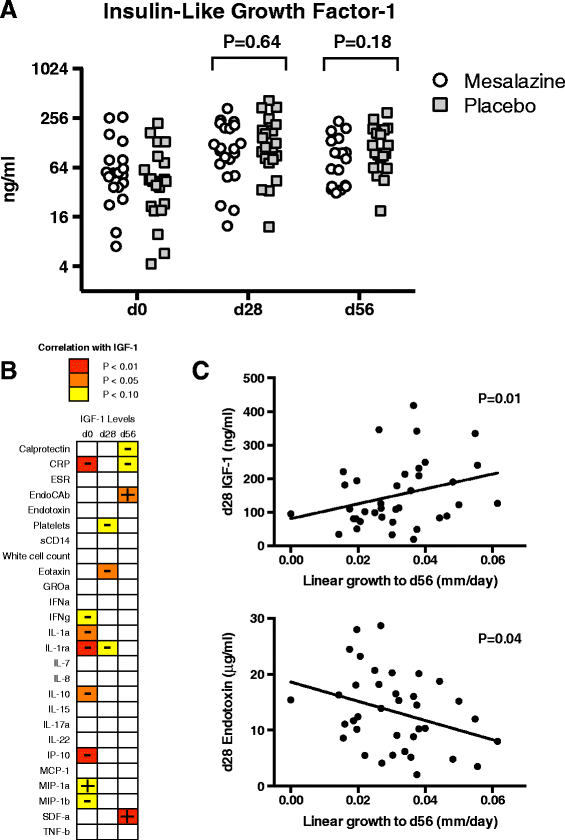
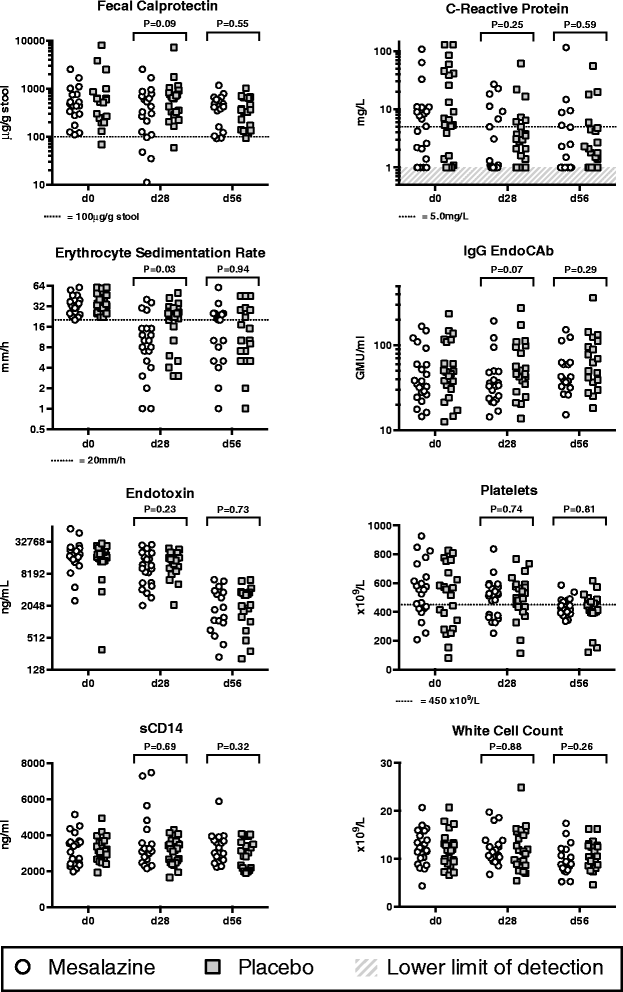
Results: Treatment with mesalazine was safe: there was no excess of adverse events, evidence of deterioration in intestinal barrier integrity or impact on nutritional recovery. There were modest reductions in several inflammatory markers with mesalazine compared to placebo. Depression of the growth hormone--insulin-like growth factor-1 axis was evident at enrollment and associated with inflammatory activation. Increases in the former and decreases in the latter correlated with linear growth.
Conclusions: Intestinal inflammation in EED is non-essential for mucosal homeostasis and is at least partly maladaptive. Further trials of gut-specific immunomodulatory therapies targeting host inflammatory activation in order to optimize the growth benefits of nutritional rehabilitation and to address stunting are warranted. Funded by The Wellcome Trust.
Trial registration: Registered at Clinicaltrials.gov NCT01841099.
Figures

Comment in
-
Environmental enteropathy and malnutrition: do we know enough to intervene?BMC Med. 2014 Oct 14;12:187. doi: 10.1186/s12916-014-0187-1. BMC Med. 2014. PMID: 25604120 Free PMC article.
References
-
- Keusch GT, Rosenberg IH, Denno DM, Duggan C, Guerrant RL, Lavery JV, Tarr PI, Ward HD, Black RE, Nataro JP, Ryan ET, Bhutta ZA, Coovadia H, Lima A, Ramakrishna B, Zaidi AK, Burgess DC, Brewer T. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low and middle-income countries. Food Nutr Bull. 2013;34:357–364. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

