Diagnostic accuracy of routine antenatal determination of fetal RHD status across gestation: population based cohort study
- PMID: 25190055
- PMCID: PMC4154470
- DOI: 10.1136/bmj.g5243
Diagnostic accuracy of routine antenatal determination of fetal RHD status across gestation: population based cohort study
Abstract
Objectives: To assess the accuracy of fetal RHD genotyping using cell-free fetal DNA in maternal plasma at different gestational ages.
Design: A prospective multicentre cohort study.
Setting: Seven maternity units in England.
Participants: RhD negative pregnant women who booked for antenatal care before 24 weeks' gestation.
Interventions: Women who gave consent for fetal RHD genotyping had blood taken at the time of booking for antenatal care and, when possible, at other routine visits such as for Down's syndrome screening between 11 and 21 weeks' gestation, at the anomaly scan at 18-21 weeks, and in the third trimester when blood was taken for the routine antibody check. The results of cord blood analysis, done routinely in RhD negative pregnancies, were also obtained to confirm the fetal RHD genotyping.
Main outcome measures: The accuracy of fetal RHD genotyping compared with RhD status predicted by cord blood serology.
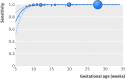
Results: Up to four analyses per woman were performed in 2288 women, generating 4913 assessable fetal results. Sensitivity for detection of fetal RHD positivity was 96.85% (94.95% to 98.05%), 99.83% (99.06% to 99.97%), 99.67% (98.17% to 99.94%), 99.82% (98.96% to 99.97%), and 100% (99.59% to 100%) at <11, 11-13, 14-17, 18-23, and >23 completed weeks' gestation, respectively. Before 11 weeks' gestation 16/865 (1.85%) babies tested were falsely predicted as RHD negative.
Conclusions: Mass throughput fetal RHD genotyping is sufficiently accurate for the prediction of RhD type if it is performed from 11 weeks' gestation. Testing before this time could result in a small but significant number of babies being incorrectly classified as RHD negative. These mothers would not receive anti-RhD immunoglobulin, and there would be a risk of haemolytic disease of the newborn in subsequent pregnancies.
© Chitty et al 2014.
Conflict of interest statement
Funding: This study was funded by the National Institute for Health Research, Research for Patient Benefit Programme (PB-PG-0107-12005), and Howard Ostin Trust (Project No 011). LSC is partially funded by the Great Ormond Street Hospital Children’s Charity and the NIHR Biomedical Research Centre at Great Ormond Street Hospital. The funded research is independent and the views expressed in the paper are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures

Comment in
-
Anti-RhD prophylaxis for RhD negative pregnant women.BMJ. 2014 Sep 4;349:g5437. doi: 10.1136/bmj.g5437. BMJ. 2014. PMID: 25190205 No abstract available.
References
-
- National Institute for Health and Care Excellence. Technology appraisal guidance 41. Guidance on the use of routine antenatal anti-D prophylaxis for RhD-negative women. NICE, 2002.
-
- Daniels G. Human blood groups. 3rd ed. Wiley-Blackwell Science, 2013.
-
- Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet 1997;350:485-7. - PubMed
-
- Daniels G, Finning K, Martin P, Massey E. Non-invasive prenatal diagnosis of fetal blood group phenotypes: current practice and future prospects. Prenat Diagn 2009;29:101-7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
