MEKK2 regulates paxillin ubiquitylation and localization in MDA-MB 231 breast cancer cells
- PMID: 25190348
- PMCID: PMC4467534
- DOI: 10.1042/BJ20140420
MEKK2 regulates paxillin ubiquitylation and localization in MDA-MB 231 breast cancer cells
Abstract
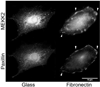
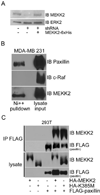
The intracellular kinase MEKK2 (mitogen-activated protein kinase/extracellular-signal-regulated kinase kinase kinase 2) is an upstream regulator of JNK (c-Jun N-terminal kinase), but additional functions for MEKK2 have not been well defined. Silencing MEKK2 expression in invasive breast tumour cells markedly inhibits xenograft metastasis, indicating that MEKK2 controls tumour cell function required for tumour progression. In our previous investigation of MEKK2 function, we discovered that tumour cell attachment to fibronectin recruits MEKK2 to focal adhesion complexes, and that MEKK2 knockdown is associated with stabilized focal adhesions and significant inhibition of tumour cell migration. In the present study we investigate MEKK2 function in focal adhesions and we report that MEKK2 physically associates with the LD1 motif of the focal adhesion protein paxillin. We reveal that MEKK2 induces paxillin ubiquitylation, and that this function requires both the paxillin LD1 motif and MEKK2 kinase activity. Finally, we demonstrate that MEKK2 promotes paxillin redistribution from focal adhesions into the cytoplasm, but does not promote paxillin degradation. Taken together, our results reveal a novel function for MEKK2 as a regulator of ubiquitylation-dependent paxillin redistribution in breast tumour cells.
Figures





References
-
- Locascio A, Nieto MA. Cell movements during vertebrate development: integrated tissue behaviour versus individual cell migration. Curr. Opin. Genet. Dev. 2001;11:464–469. - PubMed
-
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 2005;6:1182–1190. - PubMed
-
- Machesky LM. Lamellipodia and filopodia in metastasis and invasion. FEBS. Lett. 2008;582:2102–2111. - PubMed
-
- Mackay CR. Moving targets: cell migration inhibitors as new anti-inflammatory therapies. Nat. Immunol. 2008;9:988–998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

