Human vascular tissue models formed from human induced pluripotent stem cell derived endothelial cells
- PMID: 25190668
- PMCID: PMC4411213
- DOI: 10.1007/s12015-014-9549-5
Human vascular tissue models formed from human induced pluripotent stem cell derived endothelial cells
Abstract
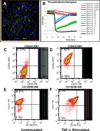
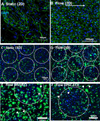
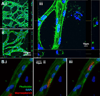
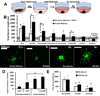
Here we describe a strategy to model blood vessel development using a well-defined induced pluripotent stem cell-derived endothelial cell type (iPSC-EC) cultured within engineered platforms that mimic the 3D microenvironment. The iPSC-ECs used here were first characterized by expression of endothelial markers and functional properties that included VEGF responsiveness, TNF-α-induced upregulation of cell adhesion molecules (MCAM/CD146; ICAM1/CD54), thrombin-dependent barrier function, shear stress-induced alignment, and 2D and 3D capillary-like network formation in Matrigel. The iPSC-ECs also formed 3D vascular networks in a variety of engineering contexts, yielded perfusable, interconnected lumen when co-cultured with primary human fibroblasts, and aligned with flow in microfluidics devices. iPSC-EC function during tubule network formation, barrier formation, and sprouting was consistent with that of primary ECs, and the results suggest a VEGF-independent mechanism for sprouting, which is relevant to therapeutic anti-angiogenesis strategies. Our combined results demonstrate the feasibility of using a well-defined, stable source of iPSC-ECs to model blood vessel formation within a variety of contexts using standard in vitro formats.
Conflict of interest statement
Figures



References
-
- Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Advanced Drug Delivery Reviews. 2011;63(4–5):300–311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

