MicroRNA detection in cervical exfoliated cells as a triage for human papillomavirus-positive women
- PMID: 25190727
- PMCID: PMC4188123
- DOI: 10.1093/jnci/dju241
MicroRNA detection in cervical exfoliated cells as a triage for human papillomavirus-positive women
Abstract
Background: Papanicolaou (Pap) triage, with high specificity, has been recommended for primary Human papillomavirus (HPV) testing but is flawed by poor sensitivity and cytologist dependence. We evaluated the potential role of microRNA (miRNA) detection in cervical exfoliated cells in HPV-positive women from a clinic-based population.
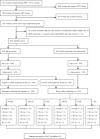
Methods: Primary HPV testing as well as Pap test were performed on all eligible women. Six miRNAs (miR-424/miR-375/miR-34a/miR-218/miR-92a/miR-93) were detected by RT-qPCR in cervical exfoliated cells. All HPV-positive women underwent colposcopy and further biopsy if indicated. Mann-Whitney U test, the receiver operating characteristic curve, logistic regression, and Pearson's Chi-square were used to assess data. All tests of statistical significance were two-sided.
Results: A total of 1021 eligible HPV-positive women were enrolled. The expression of miR-424/miR-375/miR-34a/miR-218 in high-grade cervical intraepithelial neoplasia (CIN) and abnormal cytology was statistically significantly lower than that in low-grade CIN and normal cytology, respectively (all P < .05). Compared with the Pap test, both miR-424 and miR-375 detection achieved higher sensitivity (76.0% and 74.9% vs 63.8%, P < .05), higher negative predictive value (NPV) (85.7% and 85.4% vs 79.3%, P < .05), and comparable specificity while identifying CIN2 or worse (CIN2+). Similar results were achieved while identifying CIN3+. Multi-marker panels based on miR-424, miR-375, and miR-218 further improved the performance over any single miRNA test or Pap test.
Conclusion: Single miR-424 or miR-375 detection and miR-424/miR-375/miR-218-based multimarker panels in cervical exfoliated cells show superior performance over Pap triage for high-grade CIN identification in a clinic-based population. Detection of miRNA may provide a new triage option for HPV-positive women.
© The Author 2014. Published by Oxford University Press.
Figures

References
-
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90 - PubMed
-
- Arbyn M, Castellsagué X, de Sanjosé S, et al. Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22(12):2675–2686 - PubMed
-
- Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907 - PubMed
-
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357(16):1589–1597 - PubMed
-
- Whitlock EP, Vesco KK, Eder M, et al. Liquid-based cytology and human papillomavirus testing to screen for cervical cancer: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(10):687–97, W214–W215 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

