Winter bloom of a rare betaproteobacterium in the Arctic Ocean
- PMID: 25191307
- PMCID: PMC4138443
- DOI: 10.3389/fmicb.2014.00425
Winter bloom of a rare betaproteobacterium in the Arctic Ocean
Abstract
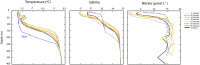
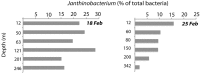
Extremely low abundance microorganisms (members of the "rare biosphere") are believed to include dormant taxa, which can sporadically become abundant following environmental triggers. Yet, microbial transitions from rare to abundant have seldom been captured in situ, and it is uncertain how widespread these transitions are. A bloom of a single ribotype (≥99% similarity in the 16S ribosomal RNA gene) of a widespread betaproteobacterium (Janthinobacterium sp.) occurred over 2 weeks in Arctic marine waters. The Janthinobacterium population was not detected microscopically in situ in January and early February, but suddenly appeared in the water column thereafter, eventually accounting for up to 20% of bacterial cells in mid February. During the bloom, this bacterium was detected at open water sites up to 50 km apart, being abundant down to more than 300 m. This event is one of the largest monospecific bacterial blooms reported in polar oceans. It is also remarkable because Betaproteobacteria are typically found only in low abundance in marine environments. In particular, Janthinobacterium were known from non-marine habitats and had previously been detected only in the rare biosphere of seawater samples, including the polar oceans. The Arctic Janthinobacterium formed mucilagenous monolayer aggregates after short (ca. 8 h) incubations, suggesting that biofilm formation may play a role in maintaining rare bacteria in pelagic marine environments. The spontaneous mass occurrence of this opportunistic rare taxon in polar waters during the energy-limited season extends current knowledge of how and when microbial transitions between rare and abundant occur in the ocean.
Keywords: Arctic; Janthinobacterium; betaproteobacteria; biofilm; bloom; rare biosphere.
Figures




References
-
- Alonso-Sáez L., Balagué V., Sa E. L., Sánchez O., González J. M., Pinhassi J., et al. (2007). Seasonality in bacterial diversity in North-west Mediterranean coastal waters: assessment through clone libraries, fingerprinting and FISH. FEMS Microbiol. Ecol. 60, 98–112 10.1111/j.1574-6941.2006.00276.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

