Cycloaddition of 1,3-butadiynes: efficient synthesis of carbo- and heterocycles
- PMID: 25191872
- PMCID: PMC6271912
- DOI: 10.3390/molecules190913788
Cycloaddition of 1,3-butadiynes: efficient synthesis of carbo- and heterocycles
Abstract
Cycloaddition reactions of alkynes are elegant, atom-efficient transformations for the synthesis of carbo- and heterocycles, mostly aromatic, involving the construction of challenging skeletons of complex molecules. Therefore significant efforts have recently been devoted to the development of novel methodologies, efficient strategies and different catalytic systems to broaden the scope of these reactions. We summarize in this review the recent advances in the cycloaddition reactions of 1,3-butadiynes to provide facile and reliable approaches to various functionalized carbo- and heterocycles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

















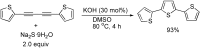




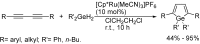
References
-
- Stiegman A.E., Graham E., Perry K.J., Khundkar L.R., Cheng L.T., Perry J.W. The electronic structure and second-order nonlinear optical properties of donor-acceptor acetylenes: A detailed investigation of structure-property relationships. J. Am. Chem. Soc. 1991;113:7658–7666. doi: 10.1021/ja00020a030. - DOI
-
- Diederich F., Rubin Y. Synthetic approaches toward molecular and polymeric carbon allotropes. Angew. Chem. Int. Ed. 1992;31:1101–1123. doi: 10.1002/anie.199211013. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

