Synaptotagmin 2 mutations cause an autosomal-dominant form of lambert-eaton myasthenic syndrome and nonprogressive motor neuropathy
- PMID: 25192047
- PMCID: PMC4157148
- DOI: 10.1016/j.ajhg.2014.08.007
Synaptotagmin 2 mutations cause an autosomal-dominant form of lambert-eaton myasthenic syndrome and nonprogressive motor neuropathy
Erratum in
- Am J Hum Genet. 2014 Oct 2;95(4):472. Gonzales, Michael [corrected to Gonzalez, Michael]
Abstract
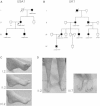
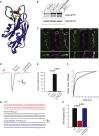
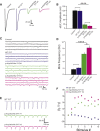
Synaptotagmin 2 is a synaptic vesicle protein that functions as a calcium sensor for neurotransmission but has not been previously associated with human disease. Via whole-exome sequencing, we identified heterozygous missense mutations in the C2B calcium-binding domain of the gene encoding Synaptotagmin 2 in two multigenerational families presenting with peripheral motor neuron syndromes. An essential calcium-binding aspartate residue, Asp307Ala, was disrupted by a c.920A>C change in one family that presented with an autosomal-dominant presynaptic neuromuscular junction disorder resembling Lambert-Eaton myasthenic syndrome. A c.923C>T variant affecting an adjacent residue (p.Pro308Leu) produced a presynaptic neuromuscular junction defect and a dominant hereditary motor neuropathy in a second family. Characterization of the mutation homologous to the human c.920A>C variant in Drosophila Synaptotagmin revealed a dominant disruption of synaptic vesicle exocytosis using this transgenic model. These findings indicate that Synaptotagmin 2 regulates neurotransmitter release at human peripheral motor nerve terminals. In addition, mutations in the Synaptotagmin 2 C2B domain represent an important cause of presynaptic congenital myasthenic syndromes and link them with hereditary motor axonopathies.
Copyright © 2014 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Littleton J.T., Stern M., Schulze K., Perin M., Bellen H.J. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993;74:1125–1134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 101876/WT_/Wellcome Trust/United Kingdom
- G1000848/MRC_/Medical Research Council/United Kingdom
- 309548/ERC_/European Research Council/International
- 096919/WT_/Wellcome Trust/United Kingdom
- ImNIH/Intramural NIH HHS/United States
- NS002824-13/NS/NINDS NIH HHS/United States
- R01 NS040296/NS/NINDS NIH HHS/United States
- G0601943/MRC_/Medical Research Council/United Kingdom
- U54 NS065712/NS/NINDS NIH HHS/United States
- MR/K000608/1/MRC_/Medical Research Council/United Kingdom
- R01 NS082563/NS/NINDS NIH HHS/United States
- G1002274/MRC_/Medical Research Council/United Kingdom
- R01 NS072248/NS/NINDS NIH HHS/United States
- R01 NS075764/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

