Crystal structure of transglutaminase 2 with GTP complex and amino acid sequence evidence of evolution of GTP binding site
- PMID: 25192068
- PMCID: PMC4156391
- DOI: 10.1371/journal.pone.0107005
Crystal structure of transglutaminase 2 with GTP complex and amino acid sequence evidence of evolution of GTP binding site
Abstract
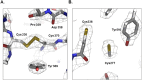
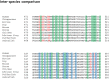
Transglutaminase2 (TG2) is a multi-functional protein involved in various cellular processes, including apoptosis, differentiation, wound healing, and angiogenesis. The malfunction of TG2 causes many human disease including inflammatory disease, celiac disease, neurodegenerative diseases, tissue fibrosis, and cancers. Protein cross-linking activity, which is representative of TG2, is activated by calcium ions and suppressed by GTP. Here, we elucidated the structure of TG2 in complex with its endogenous inhibitor, GTP. Our structure showed why GTP is the optimal nucleotide for interacting with and inhibiting TG2. In addition, sequence comparison provided information describing the evolutionary scenario of GTP usage for controlling the activity of TG2.
Conflict of interest statement
Figures

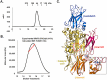



References
-
- Nemes Z Jr, Adany R, Balazs M, Boross P, Fesus L (1997) Identification of cytoplasmic actin as an abundant glutaminyl substrate for tissue transglutaminase in HL-60 and U937 cells undergoing apoptosis. J Biol Chem 272: 20577–20583. - PubMed
-
- Piacentini M, Fesus L, Farrace MG, Ghibelli L, Piredda L, et al. (1991) The expression of “tissue” transglutaminase in two human cancer cell lines is related with the programmed cell death (apoptosis). Eur J Cell Biol 54: 246–254. - PubMed
-
- Jones RA, Kotsakis P, Johnson TS, Chau DY, Ali S, et al. (2006) Matrix changes induced by transglutaminase 2 lead to inhibition of angiogenesis and tumor growth. Cell Death Differ 13: 1442–1453. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

