Utilization of inhaled corticosteroids for infants with bronchopulmonary dysplasia
- PMID: 25192252
- PMCID: PMC4156388
- DOI: 10.1371/journal.pone.0106838
Utilization of inhaled corticosteroids for infants with bronchopulmonary dysplasia
Abstract
Objective: To determine demographic and clinical variables associated with inhaled corticosteroid administration and to evaluate between-hospital variation in inhaled steroid use for infants with bronchopulmonary dysplasia (BPD).
Design: Retrospective Cohort Study.
Setting: Neonatal units of 35 US children's hospitals; as recorded in the Pediatric Health Information System (PHIS) database.
Patients: 1429 infants with evolving BPD at 28 days who were born at <29 weeks gestation with birth weight <1500 grams, admitted within the first 7 postnatal days, and discharged between January 2007-June 2011.
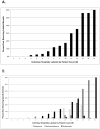
Results: Inhaled steroids were prescribed to 25% (n = 352) of the cohort with use steadily increasing during the first two months of hospitalization. The most frequently prescribed steroid was beclomethasone (n = 194, 14%), followed by budesonide (n = 125, 9%), and then fluticasone (n = 90, 6%). Birth gestation <24 weeks, birth weight 500-999 grams, and prolonged ventilation all increased the adjusted odds of ever receiving inhaled corticosteroids (p<0.05). Wide variations between hospitals in the frequency of infants ever receiving inhaled steroids (range: 0-60%) and the specific drug prescribed were noted. This variation persisted, even after controlling for observed confounders.
Conclusions: Inhaled corticosteroid administration to infants with BPD is common in neonatal units within U.S. Children's hospitals. However, its utilization varies markedly between centers from no treatment at some institutions to the majority of infants with BPD being treated at others. This supports the need for further research to identify the benefits and potential risks of inhaled steroid usage in infants with BPD.
Conflict of interest statement
Figures

References
-
- Watterberg K (2012) Evidence-based neonatal pharmacotherapy: postnatal corticosteroids. Clin Perinatol 39: 47–59. - PubMed
-
- Halliday HL, Ehrenkranz RA, Doyle LW (2010) Early (<8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev: CD001146. - PubMed
-
- Cole CH (2001) Postnatal glucocorticoid therapy for prevention of bronchopulmonary dysplasia: routes of administration compared. Semin Neonatol 6: 343–350. - PubMed
-
- Lister P, Iles R, Shaw B, Ducharme F (2000) Inhaled steroids for neonatal chronic lung disease. Cochrane Database Syst Rev: CD002311. - PubMed
-
- Onland W, Offringa M, van Kaam A (2012) Late (>/ = 7 days) inhalation corticosteroids to reduce bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev 4: CD002311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

