Multicenter study comparing case definitions used to identify patients with chronic obstructive pulmonary disease
- PMID: 25192554
- PMCID: PMC4299587
- DOI: 10.1164/rccm.201406-1166OC
Multicenter study comparing case definitions used to identify patients with chronic obstructive pulmonary disease
Abstract
Rationale: Clinical trials in chronic obstructive pulmonary disease (COPD) usually require evidence of airflow obstruction and clinical risk factors. International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes or patient-reported physician diagnoses are often used for epidemiologic studies and performance improvement programs.
Objectives: To evaluate agreement between these case definitions for COPD and to assess the comparability of study populations identified as having COPD not using the clinical trial reference standard.
Methods: We recruited patients from the COPD Outcomes-based Network for Clinical Effectiveness and Research Translation multicenter clinical registry in a cross-sectional study. Demographics, clinical, and post-bronchodilator spirometry data were collected at an in-person study visit. The kappa statistic (κ) was used to evaluate agreement. A multivariable logistic regression model was used to identify patient characteristics associated with meeting the trial reference standard.
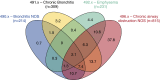
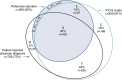
Measurements and main results: A total of 998 (82.8%) of 1,206 study participants met at least one case definition for COPD (of the 998: 91% using ICD-9 codes, 73% using patient-reported physician diagnosis, 56% using trial reference standard); agreement between case definitions was poor (κ = 0.20-0.26). Lack of airflow obstruction was the principal (89%) reason patients identified as having COPD did not meet the trial reference standard. Patients who were black (vs. white), obese (vs. normal weight), or had depression (vs. not) were less likely to meet the trial reference standard (odds ratio [95% CI], 0.37 [0.26-0.53], 0.51 [0.34-0.75], 0.53 [0.40-0.71], respectively).
Conclusions: Findings highlight concerns about the applicability of findings in clinical trials to patients meeting other case definitions for COPD.
Keywords: COPD; ICD-9-CM; case definitions; comparative effectiveness; spirometry.
Figures
Comment in
-
Can database studies be used to make the tough research decisions?Am J Respir Crit Care Med. 2014 Nov 1;190(9):967-8. doi: 10.1164/rccm.201409-1703ED. Am J Respir Crit Care Med. 2014. PMID: 25360722 No abstract available.
References
-
- National Heart Lung and Blood InstituteMorbidity & mortality: 2009 chart book on cardiovascular, lung, and blood diseases; 2011 [accessed 2014 Jun 18]. Available from: http://www.nhlbi.nih.gov/about/factbook/chapter4.htm
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)Global strategy for the diagnosis, management and prevention of chronic pulmonary disease; 2014 [accessed 2014 Jun 18]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2014_Jun11.pdf
-
- Wong GW, Miravitlles M, Chisholm A, Krishnan J. Respiratory guidelines—which real world? Ann Am Thorac Soc. 2014;11:S85–S91. - PubMed
-
- Prieto-Centurion V, Rolle AJ, Au D, Carson SS, Henderson A, Lee TA, Lindenauer PK, McBurnie M, Mularski RA, Naureckas ET, et al. A comparison of three methods used to identify patients with COPD [abstract] Am J Respir Crit Care Med. 2013;187:A5017.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

