Bayesian approach for the estimation of cyclosporine area under the curve using limited sampling strategies in pediatric hematopoietic stem cell transplantation
- PMID: 25192585
- PMCID: PMC4237955
- DOI: 10.1186/1742-4682-11-39
Bayesian approach for the estimation of cyclosporine area under the curve using limited sampling strategies in pediatric hematopoietic stem cell transplantation
Abstract
Background: The optimal marker for cyclosporine (CsA) monitoring in transplantation patients remains controversial. However, there is a growing interest in the use of the area under the concentration-time curve (AUC), particularly for cyclosporine dose adjustment in pediatric hematopoietic stem cell transplantation. In this paper, we develop Bayesian limited sampling strategies (B-LSS) for cyclosporine AUC estimation using population pharmacokinetic (Pop-PK) models and investigate related issues, with the aim to improve B-LSS prediction performance.
Methods: Twenty five pediatric hematopoietic stem cell transplantation patients receiving intravenous and oral cyclosporine were investigated. Pop-PK analyses were carried out and the predictive performance of B-LSS was evaluated using the final Pop-PK model and several related ones. The performance of B-LSS when targeting different versions of AUC was also discussed.
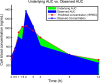
Results: A two-compartment structure model with a lag time and a combined additive and proportional error is retained. The final covariate model does not improve the B-LSS prediction performance. The best performing models for intravenous and oral cyclosporine are the structure ones with combined and additive error, respectively. Twelve B-LSS, consisting of 4 or less sampling points obtained within 4 hours post-dose, predict AUC with 95th percentile of the absolute values of relative prediction errors of 20% or less. Moreover, B-LSS perform better for the prediction of the 'underlying' AUC derived from the Pop-PK model estimated concentrations that exclude the residual errors, in comparison to their prediction of the observed AUC directly calculated using measured concentrations.
Conclusions: B-LSS can adequately estimate cyclosporine AUC. However, B-LSS performance is not perfectly in line with the standard Pop-PK model selection criteria; hence the final model might not be ideal for AUC prediction purpose. Therefore, for B-LSS application, Pop-PK model diagnostic criteria should additionally account for AUC prediction errors.
Figures



References
-
- Mahalati K, Belitsky P, Sketris I, West K, Panek R. Neoral monitoring by simplified sparse sampling area under the concentration-time curve: its relationship to acute rejection and cyclosporine nephrotoxicity early after kidney transplantation. Transplantation. 1999;68:55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

