Vitamin D deficiency leads to sensory and sympathetic denervation of the rat synovium
- PMID: 25193239
- PMCID: PMC4197091
- DOI: 10.1016/j.neuroscience.2014.08.035
Vitamin D deficiency leads to sensory and sympathetic denervation of the rat synovium
Abstract
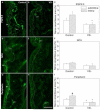
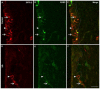
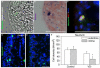
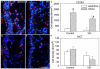
Vitamin D deficiency is associated with increased susceptibility to inflammatory arthritis. Sensory and sympathetic synovial nerves are critical to the development of inflammatory arthritis and spontaneously degenerate in the early phases of disease. These nerves contain vitamin D receptors and vitamin D influences nerve growth and neurotrophin expression. We therefore examined the density of synovial nerves and neurotrophin-containing cells in vitamin D-deficient rats. Seven-week-old Sprague-Dawley rats were fed either control or vitamin D-deficient diets for 4weeks. Knee synovium sections extending from the patella to the meniscus were immunostained for total nerves, myelinated and unmyelinated nerves, sympathetic nerves, peptidergic and non-peptidergic sensory nerves, and neurotrophins and immune cell markers. In control rats, intimal innervation by unmyelinated sensory fibers was denser than subintimal innervation. In contrast, sympathetic innervation was confined to the subintima. Many sensory axons contained markers for both peptidergic and non-peptidergic nerves. Nerve growth factor (NGF) was primarily expressed by intimal CD163-negative type B synoviocytes, while neurturin, a ligand selective for non-peptidergic sensory neurons, was expressed by synovial mast cells. In vitamin D-deficient rats, there were significant reductions in sensory nerves in the intima and sympathetic nerves in the subintima. While there was no significant change in NGF-immunoreactivity, the number of neurturin-expressing mast cells was significantly reduced in the intima, suggesting that intimal reductions in sensory nerves may be related to reductions in neurturin. Vitamin D deficiency therefore may increase susceptibility to inflammatory arthritis by depleting sensory and sympathetic synovial nerves as a result of reduced synovial neurotrophin content.
Keywords: arthritis; nerves; sensory; sympathetic; synovium; vitamin D.
Copyright © 2014 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Vitamin D deficiency promotes skeletal muscle hypersensitivity and sensory hyperinnervation.J Neurosci. 2011 Sep 28;31(39):13728-38. doi: 10.1523/JNEUROSCI.3637-11.2011. J Neurosci. 2011. PMID: 21957236 Free PMC article.
-
The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages.FASEB J. 2000 Oct;14(13):2097-107. doi: 10.1096/fj.99-1082com. FASEB J. 2000. PMID: 11023994
-
The innervation of synovium of human osteoarthritic joints in comparison with normal rat and sheep synovium.Osteoarthritis Cartilage. 2013 Sep;21(9):1383-91. doi: 10.1016/j.joca.2013.06.018. Osteoarthritis Cartilage. 2013. PMID: 23973153
-
Distribution of neuropeptide-containing nerve fibers in the synovium and adjacent bone of the rat knee joint.Clin Exp Rheumatol. 1995 Mar-Apr;13(2):173-8. Clin Exp Rheumatol. 1995. PMID: 7544709
-
Role of mast cells and sensory nerves in skin inflammation.G Ital Dermatol Venereol. 2010 Apr;145(2):195-204. G Ital Dermatol Venereol. 2010. PMID: 20467393 Review.
Cited by
-
Driving β2- While Suppressing α-Adrenergic Receptor Activity Suppresses Joint Pathology in Inflammatory Arthritis.Front Immunol. 2021 Jun 17;12:628065. doi: 10.3389/fimmu.2021.628065. eCollection 2021. Front Immunol. 2021. PMID: 34220796 Free PMC article.
-
Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders.Nutrients. 2022 Oct 17;14(20):4353. doi: 10.3390/nu14204353. Nutrients. 2022. PMID: 36297037 Free PMC article. Review.
References
-
- Abu-Amer Y, Bar-Shavit Z. Impaired bone marrow-derived macrophage differentiation in vitamin D deficiency. Cell Immunol. 1993;151:356–368. - PubMed
-
- Alexander JL, Dennerstein L, Woods NF, Halbreich U, Kotz K, Richardson G, Graziottin A, Sherman JJ. Arthralgias, bodily aches and pains and somatic complaints in midlife women: etiology, pathophysiology and differential diagnosis. Expert Rev Neurother. 2007;7:S15–26. - PubMed
-
- Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nat Rev Rheumatol. 2012;8:573–586. - PubMed
-
- Attar SM. Vitamin D deficiency in rheumatoid arthritis. Prevalence and association with disease activity in Western Saudi Arabia. Saudi Med J. 2012;33:520–525. - PubMed
-
- Bar-Shavit Z, Noff D, Edelstein S, Meyer M, Shibolet S, Goldman R. 1,25-dihydroxyvitamin D3 and the regulation of macrophage function. Calcif Tissue Int. 1981;33:673–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

