Enhanced in vivo efficacy of a type I interferon superagonist with extended plasma half-life in a mouse model of multiple sclerosis
- PMID: 25193661
- PMCID: PMC4200257
- DOI: 10.1074/jbc.M114.602474
Enhanced in vivo efficacy of a type I interferon superagonist with extended plasma half-life in a mouse model of multiple sclerosis
Abstract
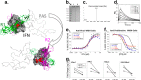
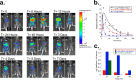
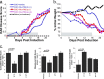
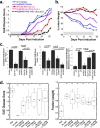
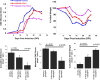
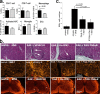
IFNβ is a common therapeutic option to treat multiple sclerosis. It is unique among the family of type I IFNs in that it binds to the interferon receptors with high affinity, conferring exceptional biological properties. We have previously reported the generation of an interferon superagonist (dubbed YNSα8) that is built on the backbone of a low affinity IFNα but modified to exhibit higher receptor affinity than even for IFNβ. Here, YNSα8 was fused with a 600-residue hydrophilic, unstructured N-terminal polypeptide chain comprising proline, alanine, and serine (PAS) to prolong its plasma half-life via "PASylation." PAS-YNSα8 exhibited a 10-fold increased half-life in both pharmacodynamic and pharmacokinetic assays in a transgenic mouse model harboring the human receptors, notably without any detectable loss in biological potency or bioavailability. This long-lived superagonist conferred significantly improved protection from MOG35-55-induced experimental autoimmune encephalomyelitis compared with IFNβ, despite being injected with a 4-fold less frequency and at an overall 16-fold lower dosage. These data were corroborated by FACS measurements showing a decrease of CD11b(+)/CD45(hi) myeloid lineage cells detectable in the CNS, as well as a decrease in IBA(+) cells in spinal cord sections determined by immunohistochemistry for PAS-YNSα8-treated animals. Importantly, PAS-YNSα8 did not induce antibodies upon repeated administration, and its biological efficacy remained unchanged after 21 days of treatment. A striking correlation between increased levels of CD274 (PD-L1) transcripts from spleen-derived CD4(+) cells and improved clinical response to autoimmune encephalomyelitis was observed, indicating that, at least in this mouse model of multiple sclerosis, CD274 may serve as a biomarker to predict the effectiveness of IFN therapy to treat this complex disease.
Keywords: Autoimmune Disease; Biomarker; Drug Development; Interferon; Multiple Sclerosis; Transgenic Mice.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Induction of endogenous Type I interferon within the central nervous system plays a protective role in experimental autoimmune encephalomyelitis.Acta Neuropathol. 2015 Jul;130(1):107-18. doi: 10.1007/s00401-015-1418-z. Epub 2015 Apr 14. Acta Neuropathol. 2015. PMID: 25869642 Free PMC article.
-
Interferon Beta-1a versus Combined Interferon Beta-1a and Oligodendrocyte-Specific FGFR1 Deletion in Experimental Autoimmune Encephalomyelitis.Int J Mol Sci. 2022 Oct 12;23(20):12183. doi: 10.3390/ijms232012183. Int J Mol Sci. 2022. PMID: 36293040 Free PMC article.
-
Bridging the species divide: transgenic mice humanized for type-I interferon response.PLoS One. 2014 Jan 9;9(1):e84259. doi: 10.1371/journal.pone.0084259. eCollection 2014. PLoS One. 2014. PMID: 24416207 Free PMC article.
-
First-line disease-modifying therapies in paediatric multiple sclerosis: a comprehensive overview.Drugs. 2012 Jun 18;72(9):1195-211. doi: 10.2165/11634010-000000000-00000. Drugs. 2012. PMID: 22642799 Review.
-
Identification of new therapeutic targets for prevention of CNS inflammation.Expert Opin Ther Targets. 2002 Apr;6(2):203-15. doi: 10.1517/14728222.6.2.203. Expert Opin Ther Targets. 2002. PMID: 12223081 Review.
Cited by
-
Engineered Human Heavy-Chain Ferritin with Half-Life Extension and Tumor Targeting by PAS and RGDK Peptide Functionalization.Pharmaceutics. 2021 Apr 9;13(4):521. doi: 10.3390/pharmaceutics13040521. Pharmaceutics. 2021. PMID: 33918853 Free PMC article.
-
STING Agonist Mitigates Experimental Autoimmune Encephalomyelitis by Stimulating Type I IFN-Dependent and -Independent Immune-Regulatory Pathways.J Immunol. 2021 May 1;206(9):2015-2028. doi: 10.4049/jimmunol.2001317. Epub 2021 Apr 5. J Immunol. 2021. PMID: 33820855 Free PMC article.
-
Recent trends in protein and peptide-based biomaterials for advanced drug delivery.Adv Drug Deliv Rev. 2020;156:133-187. doi: 10.1016/j.addr.2020.08.008. Epub 2020 Aug 29. Adv Drug Deliv Rev. 2020. PMID: 32871201 Free PMC article. Review.
-
Protein-polymer conjugation-moving beyond PEGylation.Curr Opin Chem Biol. 2015 Oct;28:181-93. doi: 10.1016/j.cbpa.2015.08.009. Epub 2015 Sep 7. Curr Opin Chem Biol. 2015. PMID: 26356631 Free PMC article. Review.
-
Control of Hepatitis B Virus by Cytokines.Viruses. 2017 Jan 20;9(1):18. doi: 10.3390/v9010018. Viruses. 2017. PMID: 28117695 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

