Mutation in the novel nuclear-encoded mitochondrial protein CHCHD10 in a family with autosomal dominant mitochondrial myopathy
- PMID: 25193783
- PMCID: PMC4796476
- DOI: 10.1007/s10048-014-0421-1
Mutation in the novel nuclear-encoded mitochondrial protein CHCHD10 in a family with autosomal dominant mitochondrial myopathy
Abstract
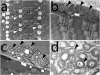
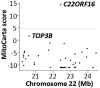
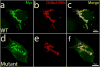
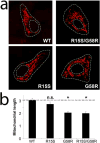
Mitochondrial myopathies belong to a larger group of systemic diseases caused by morphological or biochemical abnormalities of mitochondria. Mitochondrial disorders can be caused by mutations in either the mitochondrial or nuclear genome. Only 5% of all mitochondrial disorders are autosomal dominant. We analyzed DNA from members of the previously reported Puerto Rican kindred with an autosomal dominant mitochondrial myopathy (Heimann-Patterson et al. 1997). Linkage analysis suggested a putative locus on the pericentric region of the long arm of chromosome 22 (22q11). Using the tools of integrative genomics, we established chromosome 22 open reading frame 16 (C22orf16) (later designated as CHCHD10) as the only high-scoring mitochondrial candidate gene in our minimal candidate region. Sequence analysis revealed a double-missense mutation (R15S and G58R) in cis in CHCHD10 which encodes a coiled coil-helix-coiled coil-helix protein of unknown function. These two mutations completely co-segregated with the disease phenotype and were absent in 1,481 Caucasian and 80 Hispanic (including 32 Puerto Rican) controls. Expression profiling showed that CHCHD10 is enriched in skeletal muscle. Mitochondrial localization of the CHCHD10 protein was confirmed using immunofluorescence in cells expressing either wild-type or mutant CHCHD10. We found that the expression of the G58R, but not the R15S, mutation induced mitochondrial fragmentation. Our findings identify a novel gene causing mitochondrial myopathy, thereby expanding the spectrum of mitochondrial myopathies caused by nuclear genes. Our findings also suggest a role for CHCHD10 in the morphologic remodeling of the mitochondria.
Conflict of interest statement
Figures
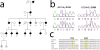

References
-
- Debray FG, Lambert M, Mitchell GA. Disorders of mitochondrial function. Curr Opin Pediatr. 2008;20:471–482. - PubMed
-
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. - PubMed
-
- Lopez MF, Kristal BS, Chernokalskaya E, Lazarev A, Shestopalov AI, Bogdanova A, Robinson M. High-throughput profiling of the mitochondrial proteome using affinity fractionation and automation. Electrophoresis. 2000;21:3427–3440. - PubMed
-
- DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. The New England journal of medicine. 2003;348:2656–2668. - PubMed
-
- Milone M, Benarroch EE. Mitochondrial dynamics: general concepts and clinical implications. Neurology. 2012;78:1612–1619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

