Erythroferrone contributes to recovery from anemia of inflammation
- PMID: 25193872
- PMCID: PMC4199959
- DOI: 10.1182/blood-2014-06-584607
Erythroferrone contributes to recovery from anemia of inflammation
Abstract
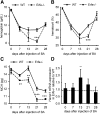
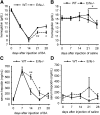
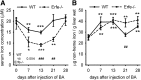
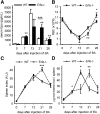
Erythroferrone (ERFE) is an erythropoiesis-driven regulator of iron homeostasis. ERFE mediates the suppression of the iron-regulatory hormone hepcidin to increase iron absorption and mobilization of iron from stores. We examined the role of ERFE in the recovery from anemia of inflammation (AI) induced by injection of heat-killed Brucella abortus. B abortus-treated wild-type mice developed a moderate anemia and reached nadir hemoglobin 14 days after injection and partially recovered by 28 days. We observed that Erfe expression in the bone marrow and the spleen was greatly increased during anemia and peaked at 14 days after injection, a time course similar to serum erythropoietin. To determine whether ERFE facilitates the recovery from anemia, we analyzed Erfe-deficient mice injected with B abortus. Compared with wild-type mice, Erfe-deficient mice exhibited a more severe anemia, had higher hepcidin levels and consequently lower serum iron concentration on days 14 and 21, and manifested impaired mobilization of iron from stores (liver and spleen). Erfe(-/-) mice eventually compensated by further stimulating erythropoiesis and reticulocyte production. Thus, ERFE contributes to the recovery from AI by suppressing hepcidin and increasing iron availability.
© 2014 by The American Society of Hematology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

