Paricalcitol for secondary hyperparathyroidism in renal transplantation
- PMID: 25194004
- PMCID: PMC4413751
- DOI: 10.1681/ASN.2013111185
Paricalcitol for secondary hyperparathyroidism in renal transplantation
Abstract
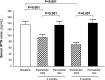
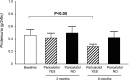
Secondary hyperparathyroidism contributes to post-transplant CKD mineral and bone disorder. Paricalcitol, a selective vitamin D receptor activator, decreased serum parathyroid hormone levels and proteinuria in patients with secondary hyperparathyroidism. This single-center, prospective, randomized, crossover, open-label study compared the effect of 6-month treatment with paricalcitol (1 μg/d for 3 months and then uptitrated to 2 µg/d if tolerated) or nonparicalcitol therapy on serum parathyroid hormone levels (primary outcome), mineral metabolism, and proteinuria in 43 consenting recipients of renal transplants with secondary hyperparathyroidism. Participants were randomized 1:1 according to a computer-generated sequence. Compared with baseline, median (interquartile range) serum parathyroid hormone levels significantly declined on paricalcitol from 115.6 (94.8-152.0) to 63.3 (52.0-79.7) pg/ml (P<0.001) but not on nonparicalcitol therapy. At 6 months, levels significantly differed between treatments (P<0.001 by analysis of covariance). Serum bone-specific alkaline phosphatase and osteocalcin decreased on paricalcitol therapy only and significantly differed between treatments at 6 months (P<0.001 for all comparisons). At 6 months, urinary deoxypyridinoline-to-creatinine ratio and 24-hour proteinuria level decreased only on paricalcitol (P<0.05). L3 and L4 vertebral mineral bone density, assessed by dual-energy x-ray absorption, significantly improved with paricalcitol at 6 months (P<0.05 for both densities). Paricalcitol was well tolerated. Overall, 6-month paricalcitol supplementation reduced parathyroid hormone levels and proteinuria, attenuated bone remodeling and mineral loss, and reduced eGFR in renal transplant recipients with secondary hyperparathyroidism. Long-term studies are needed to monitor directly measured GFR, ensure that the bone remodeling and mineral effects are sustained, and determine if the reduction in proteinuria improves renal and cardiovascular outcomes.
Keywords: hyperparathyroidism; kidney transplantation; proteinuria; vitamin D.
Copyright © 2015 by the American Society of Nephrology.
Figures

References
-
- Pichette V, Bonnardeaux A, Prudhomme L, Gagné M, Cardinal J, Ouimet D: Long-term bone loss in kidney transplant recipients: A cross-sectional and longitudinal study. Am J Kidney Dis 28: 105–114, 1996 - PubMed
-
- Julian BA, Quarles LD, Niemann KM: Musculoskeletal complications after renal transplantation: Pathogenesis and treatment. Am J Kidney Dis 19: 99–120, 1992 - PubMed
-
- Grotz WH, Mundinger FA, Gugel B, Exner V, Kirste G, Schollmeyer PJ: Bone fracture and osteodensitometry with dual energy X-ray absorptiometry in kidney transplant recipients. Transplantation 58: 912–915, 1994 - PubMed
-
- Ball AM, Gillen DL, Sherrard D, Weiss NS, Emerson SS, Seliger SL, Kestenbaum BR, Stehman-Breen C: Risk of hip fracture among dialysis and renal transplant recipients. JAMA 288: 3014–3018, 2002 - PubMed
-
- Abbott KC, Oglesby RJ, Hypolite IO, Kirk AD, Ko CW, Welch PG, Agodoa LY, Duncan WE: Hospitalizations for fractures after renal transplantation in the United States. Ann Epidemiol 11: 450–457, 2001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

