Diversification of TAM receptor tyrosine kinase function
- PMID: 25194421
- PMCID: PMC4169336
- DOI: 10.1038/ni.2986
Diversification of TAM receptor tyrosine kinase function
Abstract
The clearance of apoptotic cells is critical for both tissue homeostasis and the resolution of inflammation. We found that the TAM receptor tyrosine kinases Axl and Mer had distinct roles as phagocytic receptors in these two settings, in which they exhibited divergent expression, regulation and activity. Mer acted as a tolerogenic receptor in resting macrophages and during immunosuppression. In contrast, Axl was an inflammatory response receptor whose expression was induced by proinflammatory stimuli. Axl and Mer differed in their ligand specificities, ligand-receptor complex formation in tissues, and receptor shedding upon activation. These differences notwithstanding, phagocytosis by either protein was strictly dependent on receptor activation triggered by bridging of TAM receptor-ligand complexes to the 'eat-me' signal phosphatidylserine on the surface of apoptotic cells.
Conflict of interest statement
G.L. is a shareholder in Kolltan Pharmaceuticals.
Figures

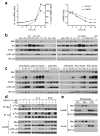
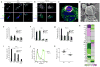
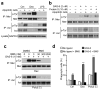


Comment in
-
Immune signalling: Waste sorting.Nat Rev Immunol. 2014 Oct;14(10):651. doi: 10.1038/nri3746. Epub 2014 Sep 19. Nat Rev Immunol. 2014. PMID: 25234147 No abstract available.
References
-
- Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol. 2010;6:280–289. - PubMed
-
- Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. - PubMed
-
- Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

