The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice
- PMID: 25194674
- PMCID: PMC4821165
- DOI: 10.1053/j.gastro.2014.08.042
The bile acid receptor TGR5 activates the TRPA1 channel to induce itch in mice
Abstract
Background & aims: Patients with cholestatic disease have increased systemic concentrations of bile acids (BAs) and profound pruritus. The G-protein-coupled BA receptor 1 TGR5 (encoded by GPBAR1) is expressed by primary sensory neurons; its activation induces neuronal hyperexcitability and scratching by unknown mechanisms. We investigated whether the transient receptor potential ankyrin 1 (TRPA1) is involved in BA-evoked, TGR5-dependent pruritus in mice.
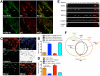
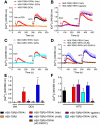
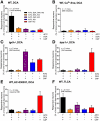
Methods: Co-expression of TGR5 and TRPA1 in cutaneous afferent neurons isolated from mice was analyzed by immunofluorescence, in situ hybridization, and single-cell polymerase chain reaction. TGR5-induced activation of TRPA1 was studied in in HEK293 cells, Xenopus laevis oocytes, and primary sensory neurons by measuring Ca(2+) signals. The contribution of TRPA1 to TGR5-induced release of pruritogenic neuropeptides, activation of spinal neurons, and scratching behavior were studied using TRPA1 antagonists or Trpa1(-/-) mice.
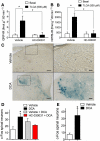
Results: TGR5 and TRPA1 protein and messenger RNA were expressed by cutaneous afferent neurons. In HEK cells, oocytes, and neurons co-expressing TGR5 and TRPA1, BAs caused TGR5-dependent activation and sensitization of TRPA1 by mechanisms that required Gβγ, protein kinase C, and Ca(2+). Antagonists or deletion of TRPA1 prevented BA-stimulated release of the pruritogenic neuropeptides gastrin-releasing peptide and atrial natriuretic peptide B in the spinal cord. Disruption of Trpa1 in mice blocked BA-induced expression of Fos in spinal neurons and prevented BA-stimulated scratching. Spontaneous scratching was exacerbated in transgenic mice that overexpressed TRG5. Administration of a TRPA1 antagonist or the BA sequestrant colestipol, which lowered circulating levels of BAs, prevented exacerbated spontaneous scratching in TGR5 overexpressing mice.
Conclusions: BAs induce pruritus in mice by co-activation of TGR5 and TRPA1. Antagonists of TGR5 and TRPA1, or inhibitors of the signaling mechanism by which TGR5 activates TRPA1, might be developed for treatment of cholestatic pruritus.
Keywords: Itching; Liver; Mouse Model; Signal Transduction.
Copyright © 2014 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Ikoma A, Steinhoff M, Stander S, et al. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

