Exendin-4 ameliorates cardiac ischemia/reperfusion injury via caveolae and caveolins-3
- PMID: 25194961
- PMCID: PMC4172825
- DOI: 10.1186/s12933-014-0132-9
Exendin-4 ameliorates cardiac ischemia/reperfusion injury via caveolae and caveolins-3
Abstract
Background: Exendin-4, an exogenous glucagon-like peptide-1 receptor (GLP-1R) agonist, protects the heart from ischemia/reperfusion injury. However, the mechanisms for this protection are poorly understood. Caveolae, sarcolemmal invaginations, and caveolins, scaffolding proteins in caveolae, localize molecules involved in cardiac protection. We tested the hypothesis that caveolae and caveolins are essential for exendin-4 induced cardiac protection using in vitro and in vivo studies in control and caveolin-3 (Cav-3) knockout mice (Cav-3 KO).
Methods: Myocytes were treated with exendin-4 and then incubated with methyl-β-cyclodextrin (MβCD) to disrupt caveolae formation. This was then followed by simulated ischemia/reperfusion (SI/R). In addition, cardiac protection in vivo was assessed by measuring infarct size and cardiac troponin levels.
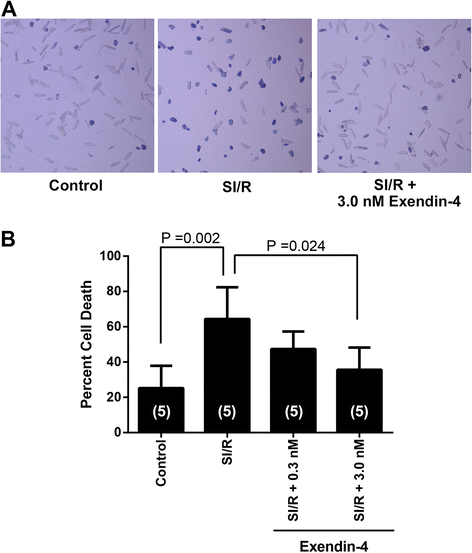
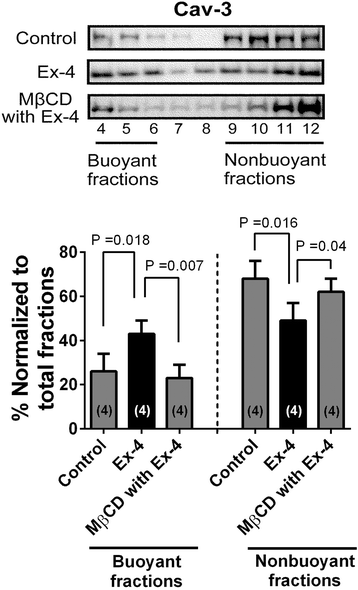
Results: Exendin-4 protected cardiac myocytes (CM) from SI/R [35.6 ± 12.6% vs. 64.4 ± 18.0% cell death, P = 0.034] and apoptosis but this protection was abolished by MβCD (71.8 ± 10.8% cell death, P = 0.004). Furthermore, Cav-3/GLP-1R co-localization was observed and membrane fractionation by sucrose density gradient centrifugation of CM treated with MβCD + exendin-4 revealed that buoyant (caveolae enriched) fractions decreased Cav-3 compared to CM treated with exendin-4 exclusively. Furthermore, exendin-4 induced a reduction in infarct size and cardiac troponin relative to control (infarct size: 25.1 ± 8.2% vs. 41.4 ± 4.1%, P < 0.001; troponin: 36.9 ± 14.2 vs. 101.1 ± 22.3 ng/ml, P < 0.001). However, exendin-4 induced cardiac protection was abolished in Cav-3 KO mice (infarct size: 43.0 ± 6.4%, P < 0.001; troponin: 96.8 ± 26.6 ng/ml, P = 0.001).
Conclusions: We conclude that caveolae and caveolin-3 are critical for exendin-4 induced protection of the heart from ischemia/reperfusion injury.
Figures



Similar articles
-
TIR/BB-loop mimetic AS-1 attenuates cardiac ischemia/reperfusion injury via a caveolae and caveolin-3-dependent mechanism.Sci Rep. 2017 Mar 14;7:44638. doi: 10.1038/srep44638. Sci Rep. 2017. PMID: 28291255 Free PMC article.
-
Caveolin-3 expression and caveolae are required for isoflurane-induced cardiac protection from hypoxia and ischemia/reperfusion injury.J Mol Cell Cardiol. 2008 Jan;44(1):123-30. doi: 10.1016/j.yjmcc.2007.10.003. Epub 2007 Oct 11. J Mol Cell Cardiol. 2008. PMID: 18054955 Free PMC article.
-
Sarcolemmal cholesterol and caveolin-3 dependence of cardiac function, ischemic tolerance, and opioidergic cardioprotection.Am J Physiol Heart Circ Physiol. 2014 Sep 15;307(6):H895-903. doi: 10.1152/ajpheart.00081.2014. Epub 2014 Jul 25. Am J Physiol Heart Circ Physiol. 2014. PMID: 25063791 Free PMC article.
-
Caveolin-1/-3: therapeutic targets for myocardial ischemia/reperfusion injury.Basic Res Cardiol. 2016 Jul;111(4):45. doi: 10.1007/s00395-016-0561-6. Epub 2016 Jun 9. Basic Res Cardiol. 2016. PMID: 27282376 Review.
-
Caveolin-3: therapeutic target for diabetic myocardial ischemia/reperfusion injury.Mol Med. 2025 Feb 26;31(1):80. doi: 10.1186/s10020-025-01117-5. Mol Med. 2025. PMID: 40012041 Free PMC article. Review.
Cited by
-
Caveolin and oxidative stress in cardiac pathology.Front Physiol. 2025 Feb 18;16:1550647. doi: 10.3389/fphys.2025.1550647. eCollection 2025. Front Physiol. 2025. PMID: 40041164 Free PMC article. Review.
-
Association among weight change, glycemic control, and markers of cardiovascular risk with exenatide once weekly: a pooled analysis of patients with type 2 diabetes.Cardiovasc Diabetol. 2015 Feb 3;14:12. doi: 10.1186/s12933-014-0171-2. Cardiovasc Diabetol. 2015. PMID: 25645567 Free PMC article. Clinical Trial.
-
TIR/BB-loop mimetic AS-1 attenuates cardiac ischemia/reperfusion injury via a caveolae and caveolin-3-dependent mechanism.Sci Rep. 2017 Mar 14;7:44638. doi: 10.1038/srep44638. Sci Rep. 2017. PMID: 28291255 Free PMC article.
-
Leucine imparts cardioprotective effects by enhancing mTOR activity and mitochondrial fusion in a myocardial ischemia/reperfusion injury murine model.Diabetol Metab Syndr. 2021 Nov 20;13(1):139. doi: 10.1186/s13098-021-00755-z. Diabetol Metab Syndr. 2021. PMID: 34801078 Free PMC article.
-
Peptides Are Cardioprotective Drugs of the Future: The Receptor and Signaling Mechanisms of the Cardioprotective Effect of Glucagon-like Peptide-1 Receptor Agonists.Int J Mol Sci. 2024 Apr 30;25(9):4900. doi: 10.3390/ijms25094900. Int J Mol Sci. 2024. PMID: 38732142 Free PMC article. Review.
References
-
- Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117(18):2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

