Why do lesions in the rodent anterior thalamic nuclei cause such severe spatial deficits?
- PMID: 25195980
- PMCID: PMC4462592
- DOI: 10.1016/j.neubiorev.2014.08.013
Why do lesions in the rodent anterior thalamic nuclei cause such severe spatial deficits?
Abstract
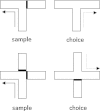
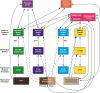
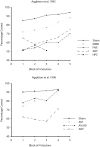
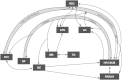
Lesions of the rodent anterior thalamic nuclei cause severe deficits to multiple spatial learning tasks. Possible explanations for these effects are examined, with particular reference to T-maze alternation. Anterior thalamic lesions not only impair allocentric place learning but also disrupt other spatial processes, including direction learning, path integration, and relative length discriminations, as well as aspects of nonspatial learning, e.g., temporal discriminations. Working memory tasks, such as T-maze alternation, appear particularly sensitive as they combine an array of these spatial and nonspatial demands. This sensitivity partly reflects the different functions supported by individual anterior thalamic nuclei, though it is argued that anterior thalamic lesion effects also arise from covert pathology in sites distal to the thalamus, most critically in the retrosplenial cortex and hippocampus. This two-level account, involving both local and distal lesion effects, explains the range and severity of the spatial deficits following anterior thalamic lesions. These findings highlight how the anterior thalamic nuclei form a key component in a series of interdependent systems that support multiple spatial functions.
Keywords: Alternation; Amnesia; Direction; Fornix; Learning; Mammillary bodies; Memory; Navigation; Space; Thalamus.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Aggleton J.P., Sahgal A. The contribution of the anterior thalamic nuclei to anterograde amnesia. Neuropsychologia. 1993;31:1001–1019. - PubMed
-
- Aggleton J.P., Hunt P.R., Rawlins J.N. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav. Brain Res. 1986;19:133–146. - PubMed
-
- Aggleton J.P., Hunt P.R., Shaw C. The effects of mammillary body and combined amygdalar-fornix lesions on tests of delayed non-matching-to-sample in the rat. Behav. Brain Res. 1990;15:145–157. - PubMed
-
- Aggleton J.P., Keith A.B., Sahgal A. Both fornix and anterior thalamic, but not mammillary, lesions disrupt delayed nonmatching-to-position memory in rats. Behav. Brain Res. 1991;44:151–161. - PubMed
-
- Aggleton J.P., Neave N., Nagle S., Hunt P.R. A comparison of the effects of anterior thalamic, mamillary body and fornix lesions on reinforced spatial alternation. Behav. Brain Res. 1995;68:91–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

