Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis
- PMID: 25196020
- PMCID: PMC4277680
- DOI: 10.1056/NEJMoa1407426
Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis
Abstract
Background: Early-phase and preclinical studies suggest that moxifloxacin-containing regimens could allow for effective 4-month treatment of uncomplicated, smear-positive pulmonary tuberculosis.
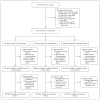
Methods: We conducted a randomized, double-blind, placebo-controlled, phase 3 trial to test the noninferiority of two moxifloxacin-containing regimens as compared with a control regimen. One group of patients received isoniazid, rifampin, pyrazinamide, and ethambutol for 8 weeks, followed by 18 weeks of isoniazid and rifampin (control group). In the second group, we replaced ethambutol with moxifloxacin for 17 weeks, followed by 9 weeks of placebo (isoniazid group), and in the third group, we replaced isoniazid with moxifloxacin for 17 weeks, followed by 9 weeks of placebo (ethambutol group). The primary end point was treatment failure or relapse within 18 months after randomization.
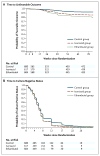
Results: Of the 1931 patients who underwent randomization, in the per-protocol analysis, a favorable outcome was reported in fewer patients in the isoniazid group (85%) and the ethambutol group (80%) than in the control group (92%), for a difference favoring the control group of 6.1 percentage points (97.5% confidence interval [CI], 1.7 to 10.5) versus the isoniazid group and 11.4 percentage points (97.5% CI, 6.7 to 16.1) versus the ethambutol group. Results were consistent in the modified intention-to-treat analysis and all sensitivity analyses. The hazard ratios for the time to culture negativity in both solid and liquid mediums for the isoniazid and ethambutol groups, as compared with the control group, ranged from 1.17 to 1.25, indicating a shorter duration, with the lower bounds of the 95% confidence intervals exceeding 1.00 in all cases. There was no significant difference in the incidence of grade 3 or 4 adverse events, with events reported in 127 patients (19%) in the isoniazid group, 111 (17%) in the ethambutol group, and 123 (19%) in the control group.
Conclusions: The two moxifloxacin-containing regimens produced a more rapid initial decline in bacterial load, as compared with the control group. However, noninferiority for these regimens was not shown, which indicates that shortening treatment to 4 months was not effective in this setting. (Funded by the Global Alliance for TB Drug Development and others; REMoxTB ClinicalTrials.gov number, NCT00864383.).
Figures
Comment in
-
Shortening treatment for tuberculosis--to basics.N Engl J Med. 2014 Oct 23;371(17):1642-3. doi: 10.1056/NEJMe1410977. N Engl J Med. 2014. PMID: 25337754 No abstract available.
-
Shorter moxifloxacin-based regimens for drug-sensitive tuberculosis.N Engl J Med. 2015 Feb 5;372(6):577. doi: 10.1056/NEJMc1414718. N Engl J Med. 2015. PMID: 25651256 No abstract available.
-
Shorter moxifloxacin-based regimens for drug-sensitive tuberculosis.N Engl J Med. 2015 Feb 5;372(6):576. doi: 10.1056/NEJMc1414718. N Engl J Med. 2015. PMID: 25651257 No abstract available.
-
Shorter moxifloxacin-based regimens for drug-sensitive tuberculosis.N Engl J Med. 2015 Feb 5;372(6):577. doi: 10.1056/NEJMc1414718. N Engl J Med. 2015. PMID: 25651258 No abstract available.
-
Four-month fluoroquinolone-containing regimens are inferior to standard 6-month tuberculosis treatment.Evid Based Med. 2015 Aug;20(4):128-9. doi: 10.1136/ebmed-2014-110139. Epub 2015 Apr 29. Evid Based Med. 2015. PMID: 25926523 No abstract available.
References
-
- Gillespie SH, Billington O. Activity of moxifloxacin against mycobacteria. J Antimicrob Chemother. 1999;44:393–5. - PubMed
-
- Falzon D, Jaramillo E, Schünemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38:516–28. - PubMed
-
- Ginsberg AM. Tuberculosis drug development: progress, challenges, and the road ahead. Tuberculosis (Edinb) 2010;90:162–7. - PubMed
-
- Grossman RF, Hsueh P-R, Gillespie SH, Blasi F. Community-acquired pneumonia and tuberculosis: differential diagnosis and the use of fluoroquinolones. Int J Infect Dis. 2014;18:14–21. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 1U01AI069469/AI/NIAID NIH HHS/United States
- U01 AI069453/AI/NIAID NIH HHS/United States
- UM1AI068634/AI/NIAID NIH HHS/United States
- 1U01AI069426/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- MC_U122888469/MRC_/Medical Research Council/United Kingdom
- 1U01AI069453/AI/NIAID NIH HHS/United States
- UM1AI106701/AI/NIAID NIH HHS/United States
- U01 AI069469/AI/NIAID NIH HHS/United States
- U01 AI069426/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
