One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities
- PMID: 25197083
- PMCID: PMC4169972
- DOI: 10.1073/pnas.1323705111
One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities
Abstract
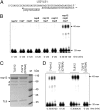
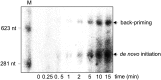
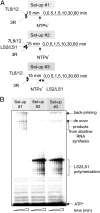
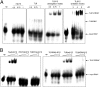
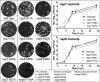
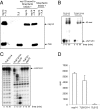
In addition to members causing milder human infections, the Coronaviridae family includes potentially lethal zoonotic agents causing severe acute respiratory syndrome (SARS) and the recently emerged Middle East respiratory syndrome. The ∼30-kb positive-stranded RNA genome of coronaviruses encodes a replication/transcription machinery that is unusually complex and composed of 16 nonstructural proteins (nsps). SARS-CoV nsp12, the canonical RNA-dependent RNA polymerase (RdRp), exhibits poorly processive RNA synthesis in vitro, at odds with the efficient replication of a very large RNA genome in vivo. Here, we report that SARS-CoV nsp7 and nsp8 activate and confer processivity to the RNA-synthesizing activity of nsp12. Using biochemical assays and reverse genetics, the importance of conserved nsp7 and nsp8 residues was probed. Whereas several nsp7 mutations affected virus replication to a limited extent, the replacement of two nsp8 residues (P183 and R190) essential for interaction with nsp12 and a third (K58) critical for the interaction of the polymerase complex with RNA were all lethal to the virus. Without a loss of processivity, the nsp7/nsp8/nsp12 complex can associate with nsp14, a bifunctional enzyme bearing 3'-5' exoribonuclease and RNA cap N7-guanine methyltransferase activities involved in replication fidelity and 5'-RNA capping, respectively. The identification of this tripartite polymerase complex that in turn associates with the nsp14 proofreading enzyme sheds light on how coronaviruses assemble an RNA-synthesizing machinery to replicate the largest known RNA genomes. This protein complex is a fascinating example of the functional integration of RNA polymerase, capping, and proofreading activities.
Keywords: processivity factor; replicative complex reconstitution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cameron CE, Götte M. In: Viral Genome Replication. Raney K, editor. New York: Springer; 2009.
-
- Kuhn RJ. Fields’ Virology. 5th Ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1001–1022.
-
- Lai MMC, Perlman S, Anderson LJ. Fields’ Virology. 5th Ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1305–1335.
-
- Lazarowitz SG. Fields’ Virology. 5th Ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 641–705.
-
- Domingo E, et al. Basic concepts in RNA virus evolution. FASEB J. 1996;10(8):859–864. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

