Activation of iNKT cells by a distinct constituent of the endogenous glucosylceramide fraction
- PMID: 25197085
- PMCID: PMC4169973
- DOI: 10.1073/pnas.1415357111
Activation of iNKT cells by a distinct constituent of the endogenous glucosylceramide fraction
Abstract
Invariant natural killer T (iNKT) cells are a specialized T-cell subset that recognizes lipids as antigens, contributing to immune responses in diverse disease processes. Experimental data suggests that iNKT cells can recognize both microbial and endogenous lipid antigens. Several candidate endogenous lipid antigens have been proposed, although the contextual role of specific antigens during immune responses remains largely unknown. We have previously reported that mammalian glucosylceramides (GlcCers) activate iNKT cells. GlcCers are found in most mammalian tissues, and exist in variable molecular forms that differ mainly in N-acyl fatty acid chain use. In this report, we purified, characterized, and tested the GlcCer fractions from multiple animal species. Although activity was broadly identified in these GlcCer fractions from mammalian sources, we also found activity properties that could not be reconciled by differences in fatty acid chain use. Enzymatic digestion of β-GlcCer and a chromatographic separation method demonstrated that the activity in the GlcCer fraction was limited to a rare component of this fraction, and was not contained within the bulk of β-GlcCer molecular species. Our data suggest that a minor lipid species that copurifies with β-GlcCer in mammals functions as a lipid self antigen for iNKT cells.
Keywords: CD1d; anomer; antigen presentation; dietary antigen; self-reactive.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


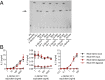
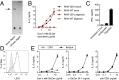
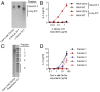

References
-
- Young MH, Gapin L. Group. 2011;1:CD1. - PubMed
-
- Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13(2):101–117. - PubMed
-
- Kawano T, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. - PubMed
-
- Kinjo Y, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434(7032):520–525. - PubMed
-
- Mattner J, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434(7032):525–529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK057521/DK/NIDDK NIH HHS/United States
- GM103422/GM/NIGMS NIH HHS/United States
- R01 AI113046/AI/NIAID NIH HHS/United States
- HHSN272201300006C/AI/NIAID NIH HHS/United States
- AI1028973/AI/NIAID NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- AI063428/AI/NIAID NIH HHS/United States
- P30 DK057521/DK/NIDDK NIH HHS/United States
- G1001750/MRC_/Medical Research Council/United Kingdom
- DK56341/DK/NIDDK NIH HHS/United States
- R01 AI063428/AI/NIAID NIH HHS/United States
- DK20579/DK/NIDDK NIH HHS/United States
- P30-CA14051/CA/NCI NIH HHS/United States
- P41 GM103422/GM/NIGMS NIH HHS/United States
- R56 AI063428/AI/NIAID NIH HHS/United States
- K08 AI102945/AI/NIAID NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- AI1007306/AI/NIAID NIH HHS/United States
- AI102945/AI/NIAID NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

