Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage
- PMID: 25197551
- PMCID: PMC4156793
- DOI: 10.1186/2045-824X-6-18
Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage
Abstract
Background: Lenvatinib is an oral inhibitor of multiple receptor tyrosine kinases (RTKs) targeting vascular endothelial growth factor receptor (VEGFR1-3), fibroblast growth factor receptor (FGFR1-4), platelet growth factor receptor α (PDGFR α), RET and KIT. Antiangiogenesis activity of lenvatinib in VEGF- and FGF-driven angiogenesis models in both in vitro and in vivo was determined. Roles of tumor vasculature (microvessel density (MVD) and pericyte coverage) as biomarkers for lenvatinib were also examined in this study.
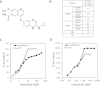
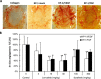
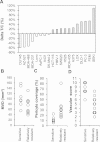
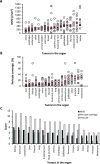
Method: We evaluated antiangiogenesis activity of lenvatinib against VEGF- and FGF-driven proliferation and tube formation of HUVECs in vitro. Effects of lenvatinib on in vivo angiogenesis, which was enhanced by overexpressed VEGF or FGF in human pancreatic cancer KP-1 cells, were examined in the mouse dorsal air sac assay. We determined antitumor activity of lenvatinib in a broad panel of human tumor xenograft models to test if vascular score, which consisted of high MVD and low pericyte coverage, was associated with sensitivity to lenvatinib treatment. Vascular score was also analyzed using human tumor specimens with 18 different types of human primary tumors.
Result: Lenvatinib inhibited VEGF- and FGF-driven proliferation and tube formation of HUVECs in vitro. In vivo angiogenesis induced by overexpressed VEGF (KP-1/VEGF transfectants) or FGF (KP-1/FGF transfectants) was significantly suppressed with oral treatments of lenvatinib. Lenvatinib showed significant antitumor activity in KP-1/VEGF and five 5 of 7 different types of human tumor xenograft models at between 1 to 100 mg/kg. We divided 19 human tumor xenograft models into lenvatinib-sensitive (tumor-shrinkage) and relatively resistant (slow-growth) subgroups based on sensitivity to lenvatinib treatments at 100 mg/kg. IHC analysis showed that vascular score was significantly higher in sensitive subgroup than relatively resistant subgroup (p < 0.0004). Among 18 types of human primary tumors, kidney cancer had the highest MVD, while liver cancer had the lowest pericyte coverage, and cancers in Kidney and Stomach had highest vascular score.
Conclusion: These results indicated that Lenvatinib inhibited VEGF- and FGF-driven angiogenesis and showed a broad spectrum of antitumor activity with a wide therapeutic window. MVD and pericyte-coverage of tumor vasculature might be biomarkers and suggest cases that would respond for lenvatinib therapy.
Keywords: FGFR kinase inhibitor; Lenvatinib; Microvessel density; Pericyte coverage; VEGFR2 kinase inhibitor.
Figures

References
-
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

