Two distinct synchronization processes in the transition to sleep: a high-density electroencephalographic study
- PMID: 25197810
- PMCID: PMC4173919
- DOI: 10.5665/sleep.4070
Two distinct synchronization processes in the transition to sleep: a high-density electroencephalographic study
Abstract
Objectives: To assess how the characteristics of slow waves and spindles change in the falling-asleep process.
Design: Participants undergoing overnight high-density electroencephalographic recordings were awakened at 15- to 30-min intervals. One hundred forty-one falling-asleep periods were analyzed at the scalp and source level.
Setting: Sleep laboratory.
Participants: Six healthy participants.
Interventions: Serial awakenings.
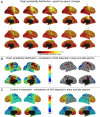
Results: The number and amplitude of slow waves followed two dissociated, intersecting courses during the transition to sleep: slow wave number increased slowly at the beginning and rapidly at the end of the falling-asleep period, whereas amplitude at first increased rapidly and then decreased linearly. Most slow waves occurring early in the transition to sleep had a large amplitude, a steep slope, involved broad regions of the cortex, predominated over frontomedial regions, and preferentially originated from the sensorimotor and the posteromedial parietal cortex. Most slow waves occurring later had a smaller amplitude and slope, involved more circumscribed parts of the cortex, and had more evenly distributed origins. Spindles were initially sparse, fast, and involved few cortical regions, then became more numerous and slower, and involved more areas.
Conclusions: Our results provide evidence for two types of slow waves, which follow dissociated temporal courses in the transition to sleep and have distinct cortical origins and distributions. We hypothesize that these two types of slow waves result from two distinct synchronization processes: (1) a "bottom-up," subcorticocortical, arousal system-dependent process that predominates in the early phase and leads to type I slow waves, and (2) a "horizontal," corticocortical synchronization process that predominates in the late phase and leads to type II slow waves. The dissociation between these two synchronization processes in time and space suggests that they may be differentially affected by experimental manipulations and sleep disorders.
Keywords: falling asleep; high-density EEG; neuronal synchronization; sleep; sleep-wake transition; slow waves; source modeling; spindles.
© 2014 Associated Professional Sleep Societies, LLC.
Figures






References
-
- Jones BE. From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci. 2005;26:578–86. - PubMed
-
- Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101:243–76. - PubMed
-
- Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull. 2006;69:86–94. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

