A small library of synthetic di-substituted 1, 4-naphthoquinones induces ROS-mediated cell death in murine fibroblasts
- PMID: 25197824
- PMCID: PMC4157788
- DOI: 10.1371/journal.pone.0106828
A small library of synthetic di-substituted 1, 4-naphthoquinones induces ROS-mediated cell death in murine fibroblasts
Erratum in
- PLoS One. 2014;9(12):e116133. Estrada, Abril [added]; Li, Qingyi [added]; Martinez, Luis [added]
Abstract
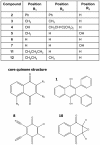
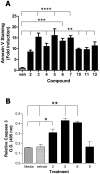
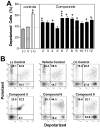
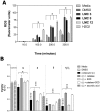
Synthesis of compound libraries and their concurrent assessment as selective reagents for probing and modulating biological function continues to be an active area of chemical biology. Microwave-assisted solid-phase Dötz benzannulation reactions have been used to inexpensively synthesize 2, 3-disubstituted-1, 4-naphthoquinone derivatives. Herein, we report the biological testing of a small library of such compounds using a murine fibroblast cell line (L929). Assessment of cellular viability identified three categories of cytotoxic compounds: no toxicity, low/intermediate toxicity and high toxicity. Increased levels of Annexin-V-positive staining and of caspase 3 activity confirmed that low, intermediate, and highly toxic compounds promote cell death. The compounds varied in their ability to induce mitochondrial depolarization and formation of reactive oxygen species (ROS). Both cytotoxic and non-cytotoxic compounds triggered mitochondrial depolarization, while one highly cytotoxic compound did not. In addition, all cytotoxic compounds promoted increased intracellular ROS but the cells were only partially protected from compound-induced apoptosis when in the presence of superoxide dismutase, catalase, or ascorbic acid suggesting utilization of additional pro-death mechanisms. In summary, nine of twelve (75%) 1, 4-naphthoquinone synthetic compounds were cytotoxic. Although the mitochondria did not appear to be a central target for induction of cell death, all of the cytotoxic compounds induced ROS formation. Thus, the data demonstrate that the synthesis regime effectively created cytotoxic compounds highlighting the potential use of the regime and its products for the identification of biologically relevant reagents.
Conflict of interest statement
Figures


Similar articles
-
Plumbagin, a plant-derived naphthoquinone metabolite induces mitochondria mediated apoptosis-like cell death in Leishmania donovani: an ultrastructural and physiological study.Apoptosis. 2016 Aug;21(8):941-53. doi: 10.1007/s10495-016-1259-9. Apoptosis. 2016. PMID: 27315817
-
Differential cellular responses to protein adducts of naphthoquinone and monocrotaline pyrrole.Chem Res Toxicol. 2010 Sep 20;23(9):1504-13. doi: 10.1021/tx1002436. Chem Res Toxicol. 2010. PMID: 20695460 Free PMC article.
-
Cytotoxicity and apoptosis induced by a plumbagin derivative in estrogen positive MCF-7 breast cancer cells.Anticancer Agents Med Chem. 2014 Jan;14(1):170-80. doi: 10.2174/18715206113136660369. Anticancer Agents Med Chem. 2014. PMID: 24164046 Free PMC article.
-
Novel multiple apoptotic mechanism of shikonin in human glioma cells.Ann Surg Oncol. 2012 Sep;19(9):3097-106. doi: 10.1245/s10434-012-2324-4. Epub 2012 Mar 24. Ann Surg Oncol. 2012. PMID: 22446899
-
Advance in Anti-tumor Mechanisms of Shikonin, Alkannin and their Derivatives.Mini Rev Med Chem. 2018;18(2):164-172. doi: 10.2174/1389557517666170228114809. Mini Rev Med Chem. 2018. PMID: 28245783 Review.
Cited by
-
Cytotoxic and mutagenic potential of juglone: a comparison of free and nano-encapsulated form.Arh Hig Rada Toksikol. 2020 Mar 1;71(1):69-77. doi: 10.2478/aiht-2020-71-3344. Arh Hig Rada Toksikol. 2020. PMID: 32597139 Free PMC article.
-
Copper-Plumbagin Complex Produces Potent Anticancer Effects by Depolymerizing Microtubules and Inducing Reactive Oxygen Species and DNA Damage.ACS Omega. 2023 Jan 10;8(3):3221-3235. doi: 10.1021/acsomega.2c06691. eCollection 2023 Jan 24. ACS Omega. 2023. PMID: 36713695 Free PMC article.
-
Pharmacological Features and Therapeutic Implications of Plumbagin in Cancer and Metabolic Disorders: A Narrative Review.Nutrients. 2024 Sep 8;16(17):3033. doi: 10.3390/nu16173033. Nutrients. 2024. PMID: 39275349 Free PMC article. Review.
-
3D-Printed PCL Scaffolds Combined with Juglone for Skin Tissue Engineering.Bioengineering (Basel). 2022 Aug 30;9(9):427. doi: 10.3390/bioengineering9090427. Bioengineering (Basel). 2022. PMID: 36134974 Free PMC article.
-
Reprogramming of leukemic cell metabolism through the naphthoquinonic compound Quambalarine B.Oncotarget. 2017 Oct 7;8(61):103137-103153. doi: 10.18632/oncotarget.21663. eCollection 2017 Nov 28. Oncotarget. 2017. PMID: 29262552 Free PMC article.
References
-
- Bentinger M, Tekle M, Dallner G (2010) Coenzyme Q – Biosynthesis and functions. . Biochemical and Biophysical Research Communications 396(1) p. 74–79. - PubMed
-
- Kim YM, Lee CH, Kim HG, Lee HS (2004) Anthraquinones Isolated from Cassia tora (Leguminosae) Seed Show an Antifungal Property against Phytopathogenic Fungi. . Journal of Agricultural and Food Chemistry 52(20) p. 6096–6100. - PubMed
-
- Tasdemir D, Brun R, Yardley V, Franzblau SG, Ruedi P (2006) Antituberculotic and Antiprotozoal Activities of Primin, a Natural Benzoquinone: In vitro and in vivo Studies. . Chemistry & Biodiversity 3(11) p. 1230–1237. - PubMed
-
- Ui H, Ishiyama A, Sekiguchi H, Namatame M, Nishihara A, et al. (2007) Selective and Potent In Vitro Antimalarial Activities Found in Four Microbial Metabolites. . The Journal of Antibiotics 60(3) p. 220–222. - PubMed
-
- Brondani DJ, Nascimento CR, de M Moreira DR, Lima Leite AC, de Souza IA, et al. (2007) Synthesis and Antitumour Activity of the Primin (2-methoxy-6-n-pentyl-1, 4-benzoquinone) and Analogues. . Medicinal Chemistry 3(4) p. 369–372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

