Sorcin, a calcium binding protein involved in the multidrug resistance mechanisms in cancer cells
- PMID: 25197934
- PMCID: PMC6271628
- DOI: 10.3390/molecules190913976
Sorcin, a calcium binding protein involved in the multidrug resistance mechanisms in cancer cells
Abstract
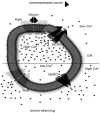
Sorcin is a penta-EF hand calcium binding protein, which participates in the regulation of calcium homeostasis in cells. Sorcin regulates calcium channels and exchangers located at the plasma membrane and at the endo/sarcoplasmic reticulum (ER/SR), and allows high levels of calcium in the ER to be maintained, preventing ER stress and possibly, the unfolded protein response. Sorcin is highly expressed in the heart and in the brain, and overexpressed in many cancer cells. Sorcin gene is in the same amplicon as other genes involved in the resistance to chemotherapeutics in cancer cells (multi-drug resistance, MDR) such as ABCB4 and ABCB1; its overexpression results in increased drug resistance to a number of chemotherapeutic agents, and inhibition of sorcin expression by sorcin-targeting RNA interference leads to reversal of drug resistance. Sorcin is increasingly considered a useful marker of MDR and may represent a therapeutic target for reversing tumor multidrug resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kawasaki H., Kretsinger R.H. Calcium-binding proteins 1: EF-hands. Protein Profile. 1995;2:297–490. - PubMed
-
- Ilari A., Johnson K.A., Nastopoulos V., Verzili D., Zamparelli C., Colotti G., Tsernoglou D., Chiancone E. The crystal structure of the sorcin calcium binding domain provides a model of Ca2+-dependent processes in the full-length protein. J. Mol. Biol. 2002;317:447–458. doi: 10.1006/jmbi.2002.5417. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

