A galactose-binding lectin isolated from Aplysia kurodai (sea hare) eggs inhibits streptolysin-induced hemolysis
- PMID: 25197935
- PMCID: PMC6271371
- DOI: 10.3390/molecules190913990
A galactose-binding lectin isolated from Aplysia kurodai (sea hare) eggs inhibits streptolysin-induced hemolysis
Erratum in
-
Correction: Hasan, I., et al. A Galactose-Binding Lectin Isolated from Aplysia kurodai (Sea Hare) Eggs Inhibits Streptolysin-Induced Hemolysis. Molecules 2014, 19, 13990-14003.Molecules. 2016 Jan 21;21(1):129. doi: 10.3390/molecules21010129. Molecules. 2016. PMID: 26791293 Free PMC article. No abstract available.
Abstract
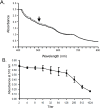
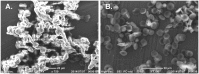
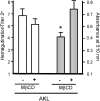
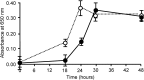
A specific galactose-binding lectin was shown to inhibit the hemolytic effect of streptolysin O (SLO), an exotoxin produced by Streptococcus pyogenes. Commercially available lectins that recognize N-acetyllactosamine (ECA), T-antigen (PNA), and Tn-antigen (ABA) agglutinated rabbit erythrocytes, but had no effect on SLO-induced hemolysis. In contrast, SLO-induced hemolysis was inhibited by AKL, a lectin purified from sea hare (Aplysia kurodai) eggs that recognizes α-galactoside oligosaccharides. This inhibitory effect was blocked by the co-presence of d-galactose, which binds to AKL. A possible explanation for these findings is that cholesterol-enriched microdomains containing glycosphingolipids in the erythrocyte membrane become occupied by tightly stacked lectin molecules, blocking the interaction between cholesterol and SLO that would otherwise result in penetration of the membrane. Growth of S. pyogenes was inhibited by lectins from a marine invertebrate (AKL) and a mushroom (ABA), but was promoted by a plant lectin (ECA). Both these inhibitory and promoting effects were blocked by co-presence of galactose in the culture medium. Our findings demonstrate the importance of glycans and lectins in regulating mechanisms of toxicity, creation of pores in the target cell membrane, and bacterial growth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Shiseki M., Miwa K., Nemoto Y., Kato H., Suzuki J., Sekiya K., Murai T., Kikuchi T., Yamashita N., Totsuka K., et al. Comparison of pathogenic factors expressed by group A Streptococci isolated from patients with streptococcal toxic shock syndrome and scarlet fever. Microb. Pathog. 1999;27:243–252. doi: 10.1006/mpat.1999.0302. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

