Cost-effectiveness of treatment strategies for BRAF-mutated metastatic melanoma
- PMID: 25198196
- PMCID: PMC4157865
- DOI: 10.1371/journal.pone.0107255
Cost-effectiveness of treatment strategies for BRAF-mutated metastatic melanoma
Erratum in
-
Correction: Cost-Effectiveness Of Treatment Strategies for BRAF-Mutated Metastatic Melanoma.PLoS One. 2015 Apr 20;10(4):e0126988. doi: 10.1371/journal.pone.0126988. eCollection 2015. PLoS One. 2015. PMID: 25893993 Free PMC article. No abstract available.
Abstract
Purpose: Genetically-targeted therapies are both promising and costly advances in the field of oncology. Several treatments for metastatic melanoma with a mutation in the BRAF gene have been approved. They extend life but are more expensive than the previous standard of care (dacarbazine). Vemurafenib, the first drug in this class, costs $13,000 per month ($207,000 for a patient with median survival). Patients failing vemurafenib are often given ipilimumab, an immunomodulator, at $150,000 per course. Assessment of cost-effectiveness is a valuable tool to help navigate the transition toward targeted cancer therapy.
Methods: We performed a cost-utility analysis to compare three strategies for patients with BRAF+ metastatic melanoma using a deterministic expected-value decision tree model to calculate the present value of lifetime costs and quality-adjusted life years (QALYs) for each strategy. We performed sensitivity analyses on all variables.
Results: In the base case, the incremental cost-effectiveness ratio (ICER) for vemurafenib compared with dacarbazine was $353,993 per QALY gained (0.42 QALYs added, $156,831 added). The ICER for vemurafenib followed by ipilimumab compared with vemurafenib alone was $158,139. In sensitivity analysis, treatment cost had the largest influence on results: the ICER for vemurafenib versus dacarbazine dropped to $100,000 per QALY gained with a treatment cost of $3600 per month.
Conclusion: The cost per QALY gained for treatment of BRAF+ metastatic melanoma with vemurafenib alone or in combination exceeds widely-cited thresholds for cost-effectiveness. These strategies may become cost-effective with lower drug prices or confirmation of a durable response without continued treatment.
Conflict of interest statement
Figures
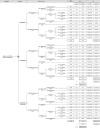
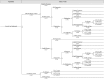
References
-
- Ray T (2014) Zelboraf Show Strong Growth in First Quarter of 2013. Pharmacogenomics Report. Available: http://www.genomeweb.com/clinical-genomics/pgx-drugs-xalkori-zelboraf-sh....
-
- Chapman PB, Einhorn LH, Meyers ML, Saxman S, Destro AN, et al. (1999) Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol Off J Am Soc Clin Oncol 17: 2745–2751. - PubMed
-
- Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, et al. (2000) Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol Off J Am Soc Clin Oncol 18: 158–166. - PubMed
-
- RED BOOK Online [internet database]. Greenwood Village, Colo: Thomson Healthcare. Updated periodically. Accessed 2013 Oct 25. (n.d.).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

