Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration
- PMID: 25198551
- PMCID: PMC4157844
- DOI: 10.1371/journal.pone.0107001
Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration
Erratum in
-
Correction: paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration.PLoS One. 2015 Mar 4;10(3):e0119262. doi: 10.1371/journal.pone.0119262. eCollection 2015. PLoS One. 2015. PMID: 25738303 Free PMC article. No abstract available.
Abstract
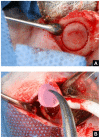
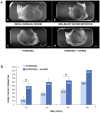
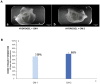
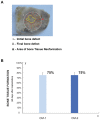
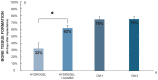
Mesenchymal stem cell (MSC) transplantation has proved to be a promising strategy in cell therapy and regenerative medicine. Although their mechanism of action is not completely clear, it has been suggested that their therapeutic activity may be mediated by a paracrine effect. The main goal of this study was to evaluate by radiographic, morphometric and histological analysis the ability of mesenchymal stem cells derived from human adipose tissue (Ad-MSC) and their conditioned medium (CM), to repair surgical bone lesions using an in vivo model (rabbit mandibles). The results demonstrated that both, Ad-MSC and CM, induce bone regeneration in surgically created lesions in rabbit's jaws, suggesting that Ad-MSC improve the process of bone regeneration mainly by releasing paracrine factors. The evidence of the paracrine effect of MSC on bone regeneration has a major impact on regenerative medicine, and the use of their CM can address some issues and difficulties related to cell transplants. In particular, CM can be easily stored and transported, and is easier to handle by medical personnel during clinical procedures.
Conflict of interest statement
Figures






References
-
- Yamachika E, Tsujigiwa H, Matsubara M, Hirata Y, Kita K, et al. (2012) Basic fibroblast growth factor supports expansion of mouse compact bone-derived mesenchymal stem cells (MSCs) and regeneration of bone from MSC in vivo. J Mol Histol 43: 223–233. - PubMed
-
- Im GI, Shin YW, Lee KB (2005) Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage 13: 845–853. - PubMed
-
- Monaco E, Bionaz M, Hollister SJ, Wheeler MB Strategies for regeneration of the bone using porcine adult adipose-derived mesenchymal stem cells. Theriogenology 75: 1381–1399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

