Glutamine supplementation for critically ill adults
- PMID: 25199493
- PMCID: PMC6517119
- DOI: 10.1002/14651858.CD010050.pub2
Glutamine supplementation for critically ill adults
Abstract
Background: Glutamine is a non-essential amino acid which is abundant in the healthy human body. There are studies reporting that plasma glutamine levels are reduced in patients with critical illness or following major surgery, suggesting that glutamine may be a conditionally essential amino acid in situations of extreme stress. In the past decade, several clinical trials examining the effects of glutamine supplementation in patients with critical illness or receiving surgery have been done, and the systematic review of this clinical evidence has suggested that glutamine supplementation may reduce infection and mortality rates in patients with critical illness. However, two recent large-scale randomized clinical trials did not find any beneficial effects of glutamine supplementation in patients with critical illness.
Objectives: The objective of this review was to:1. assess the effects of glutamine supplementation in critically ill adults and in adults after major surgery on infection rate, mortality and other clinically relevant outcomes;2. investigate potential heterogeneity across different patient groups and different routes for providing nutrition.
Search methods: We searched the Cochrane Anaesthesia Review Group (CARG) Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2013, Issue 5); MEDLINE (1950 to May 2013); EMBASE (1980 to May 2013) and Web of Science (1945 to May 2013).
Selection criteria: We included controlled clinical trials with random or quasi-random allocation that examined glutamine supplementation versus no supplementation or placebo in adults with a critical illness or undergoing elective major surgery. We excluded cross-over trials.
Data collection and analysis: Two authors independently extracted the relevant information from each included study using a standardized data extraction form. For infectious complications and mortality and morbidity outcomes we used risk ratio (RR) as the summary measure with the 95% confidence interval (CI). We calculated, where appropriate, the number needed to treat to benefit (NNTB) and the number needed to treat to harm (NNTH). We presented continuous data as the difference between means (MD) with the 95% CI.
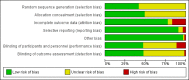
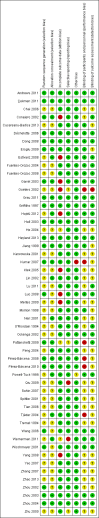
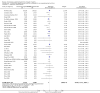
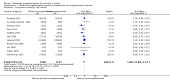
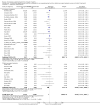
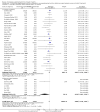
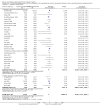
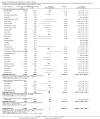
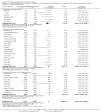
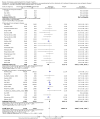
Main results: Our search identified 1999 titles, of which 53 trials (57 articles) fulfilled our inclusion criteria. The 53 included studies enrolled a total of 4671 participants with critical illness or undergoing elective major surgery. We analysed seven domains of potential risk of bias. In 10 studies the risk of bias was evaluated as low in all of the domains. Thirty-three trials (2303 patients) provided data on nosocomial infectious complications; pooling of these data suggested that glutamine supplementation reduced the infectious complications rate in adults with critical illness or undergoing elective major surgery (RR 0.79, 95% CI 0.71 to 0.87, P < 0.00001, I² = 8%, moderate quality evidence). Thirty-six studies reported short-term (hospital or less than one month) mortality. The combined rate of mortality from these studies was not statistically different between the groups receiving glutamine supplement and those receiving no supplement (RR 0.89, 95% CI 0.78 to 1.02, P = 0.10, I² = 22%, low quality evidence). Eleven studies reported long-term (more than six months) mortality; meta-analysis of these studies (2277 participants) yielded a RR of 1.00 (95% CI 0.89 to 1.12, P = 0.94, I² = 30%, moderate quality evidence). Subgroup analysis of infectious complications and mortality outcomes did not find any statistically significant differences between the predefined groups. Hospital length of stay was reported in 36 studies. We found that the length of hospital stay was shorter in the intervention group than in the control group (MD -3.46 days, 95% CI -4.61 to -2.32, P < 0.0001, I² = 63%, low quality evidence). Slightly prolonged intensive care unit (ICU) stay was found in the glutamine supplemented group from 22 studies (2285 participants) (MD 0.18 days, 95% CI 0.07 to 0.29, P = 0.002, I² = 11%, moderate quality evidence). Days on mechanical ventilation (14 studies, 1297 participants) was found to be slightly shorter in the intervention group than in the control group (MD - 0.69 days, 95% CI -1.37 to -0.02, P = 0.04, I² = 18%, moderate quality evidence). There was no clear evidence of a difference between the groups for side effects and quality of life, however results were imprecise for serious adverse events and few studies reported on quality of life. Sensitivity analysis including only low risk of bias studies found that glutamine supplementation had beneficial effects in reducing the length of hospital stay (MD -2.9 days, 95% CI -5.3 to -0.5, P = 0.02, I² = 58%, eight studies) while there was no statistically significant difference between the groups for all of the other outcomes.
Authors' conclusions: This review found moderate evidence that glutamine supplementation reduced the infection rate and days on mechanical ventilation, and low quality evidence that glutamine supplementation reduced length of hospital stay in critically ill or surgical patients. It seems to have little or no effect on the risk of mortality and length of ICU stay, however. The effects on the risk of serious side effects were imprecise. The strength of evidence in this review was impaired by a high risk of overall bias, suspected publication bias, and moderate to substantial heterogeneity within the included studies.
Conflict of interest statement
Kun‐Ming Tao: none known
Xiao‐Qian Li: none known
Li‐Qun Yang: none known
Wei‐Feng Yu: none known
Zhi‐Jie Lu: none known
Yu‐Ming Sun: none known
Fei‐Xiang Wu: none known
Figures












Comment in
-
[Intensive care: glutamine administration for critically ill patients is of limited use].Dtsch Med Wochenschr. 2015 Jan;140(1):15. doi: 10.1055/s-0040-100409. Epub 2015 Jan 12. Dtsch Med Wochenschr. 2015. PMID: 25580964 German. No abstract available.
Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia.Cochrane Database Syst Rev. 2016 Oct 25;10(10):CD008367. doi: 10.1002/14651858.CD008367.pub3. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Dec 24;12:CD008367. doi: 10.1002/14651858.CD008367.pub4. PMID: 27778318 Free PMC article. Updated.
-
Single induction dose of etomidate versus other induction agents for endotracheal intubation in critically ill patients.Cochrane Database Syst Rev. 2015 Jan 8;1(1):CD010225. doi: 10.1002/14651858.CD010225.pub2. Cochrane Database Syst Rev. 2015. PMID: 25568981 Free PMC article.
-
Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia.Cochrane Database Syst Rev. 2013 Aug 13;(8):CD008367. doi: 10.1002/14651858.CD008367.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2016 Oct 25;10:CD008367. doi: 10.1002/14651858.CD008367.pub3. PMID: 23939759 Updated.
-
Exercise rehabilitation following intensive care unit discharge for recovery from critical illness.Cochrane Database Syst Rev. 2015 Jun 22;2015(6):CD008632. doi: 10.1002/14651858.CD008632.pub2. Cochrane Database Syst Rev. 2015. PMID: 26098746 Free PMC article.
Cited by
-
A Population Pharmacokinetic Analysis of L-Glutamine Exposure in Patients with Sickle Cell Disease: Evaluation of Dose and Food Effects.Clin Pharmacokinet. 2024 Mar;63(3):357-365. doi: 10.1007/s40262-024-01349-4. Epub 2024 Feb 24. Clin Pharmacokinet. 2024. PMID: 38401036 Free PMC article. Clinical Trial.
-
Nutrition: A Primary Therapy in Pediatric Acute Respiratory Distress Syndrome.Front Pediatr. 2016 Oct 13;4:108. doi: 10.3389/fped.2016.00108. eCollection 2016. Front Pediatr. 2016. PMID: 27790606 Free PMC article. Review.
-
Glutamine in critically ill patients: is it a fundamental nutritional supplement?Rev Bras Ter Intensiva. 2016 Jun;28(2):100-3. doi: 10.5935/0103-507X.20160022. Rev Bras Ter Intensiva. 2016. PMID: 27410403 Free PMC article. No abstract available.
-
Is the glutamine story over?Crit Care. 2016 Nov 10;20(1):361. doi: 10.1186/s13054-016-1531-y. Crit Care. 2016. PMID: 27829456 Free PMC article. Review.
-
Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children.Intensive Care Med. 2020 Feb;46(Suppl 1):10-67. doi: 10.1007/s00134-019-05878-6. Intensive Care Med. 2020. PMID: 32030529 Free PMC article.
References
References to studies included in this review
Andrews 2011 {published data only}
-
- Andrews PJ, Avenell A, Noble DW, Campbell MK, Croal BL, Simpson WG, et al. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ 2011;17(342):d1542. [PUBMED: 21415104] - PubMed
Çekmen 2011 {published data only}
-
- Cekmen N, AydIn A, Erdemli Ö. The impact of L‐alanyl‐L‐glutamine dipeptide supplemented total parenteral nutrition on clinical outcome in critically patients. European e‐Journal of Clinical Nutrition and Metabolism 2011;6:e64‐7. [DOI: 10.1016/j.eclnm.2011.02.001] - DOI
Chai 2006 {published data only}
-
- Cai GL, Yan J, Yu YH, Zhang ZC, Gong SJ, Dai HW, et al. Influence of glutamine and growth hormone intensified nutrition support on immunomodulation in critically ill elderly patients. Chinese Critical Care Medicine 2006;18(10):595‐8. - PubMed
Conejero 2002 {published data only}
-
- Conejero R, Bonet A, Grau T, Esteban A, Mesejo A, Montejo JC, et al. Effect of a glutamine‐enriched enteral diet on intestinal permeability and infectious morbidity at 28 days in critically ill patients with systemic inflammatory response syndrome: a randomized, single‐blind, prospective, multicenter study. Nutrition 2002;18(9):716‐21. [PUBMED: 12297203] - PubMed
Cucereanu‐Badica 2013 {published data only}
-
- Cucereanu‐Badica I, Luca I, Negres S, Grintescu I, Lascar I. The effects of intravenous glutamine supplementation in severely burned, multiple traumatized patients. Farmacia 61;1:212‐9. [http://www.revistafarmacia.ro/201301/art‐19‐2013‐1‐cucereanu%20212‐219.pdf]
Déchelotte 2006 {published data only}
-
- Déchelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coëffier M, Hecketsweiler B, et al. L‐alanyl‐L‐glutamine dipeptide‐supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double‐blind, multicenter study. Critical Care Medicine 2006;34(3):598‐604. [PUBMED: 16505644] - PubMed
Dong 2008 {published data only}
-
- Dong GL, Kang ZH, Liu XN, Ji G, Wang CY, Wan Y, et al. Effect of alanyl‐glutamine on the clinical outcome of patients after total gastrectomy. Chinese Journal of Clinical Nutrition 2008;16(2):70‐3. [EMBASE: 2008262444]
Eroglu 2009 {published data only}
-
- Eroglu A. The effect of intravenous alanyl‐glutamine supplementation on plasma glutathione levels in intensive care unit trauma patients receiving enteral nutrition: the results of a randomized controlled trial. Anesthesia and Analgesia 2009;109(2):502‐5. [PUBMED: 19608826] - PubMed
Estívariz 2008 {published data only}
-
- Estívariz CF, Griffith DP, Luo M, Szeszycki EE, Bazargan N, Dave N, et al. Efficacy of parenteral nutrition supplemented with glutamine dipeptide to decrease hospital infections in critically ill surgical patients. Journal of Parenteral and Enteral Nutrition 2008;32(4):389‐402. [PUBMED: 18596310] - PMC - PubMed
-
- Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez‐Estivariz C, Griffith DP, et al. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Medicine 2005;31(8):1079‐86. [PUBMED: 15973519] - PubMed
Fuentes‐Orozco 2004 {published data only}
-
- Fuentes‐Orozco C, Anaya‐Prado R, González‐Ojeda A, Arenas‐Márquez H, Cabrera‐Pivaral C, Cervantes‐Guevara G, et al. L‐alanyl‐L‐glutamine‐supplemented parenteral nutrition improves infectious morbidity in secondary peritonitis. Clinical Nutrition 2004;23(1):13‐21. [PUBMED: 14757388] - PubMed
Fuentes‐Orozco 2008 {published data only}
-
- Fuentes‐Orozco C, Cervantes‐Guevara G, Muciño‐Hernández I, López‐Ortega A, Ambriz‐González G, Gutiérrez‐de‐la‐Rosa JL, et al. L‐alanyl‐L‐glutamine‐supplemented parenteral nutrition decreases infectious morbidity rate in patients with severe acute pancreatitis. Journal of Parenteral and Enteral Nutrition 2008;32(4):403‐11. [PUBMED: 18596311] - PubMed
Garrel 2003 {published data only}
-
- Garrel D, Patenaude J, Nedelec B, Samson L, Dorais J, Champoux J, et al. Decreased mortality and infectious morbidity in adult burn patients given enteral glutamine supplements: a prospective, controlled, randomized clinical trial. Critical Care Medicine 2003;31(10):2444‐9. [PUBMED: 14530749] - PubMed
Goeters 2002 {published data only}
-
- Goeters C, Wenn A, Mertes N, Wempe C, Aken H, Stehle P, et al. Parenteral L‐alanyl‐L‐glutamine improves 6‐month outcome in critically ill patients. Critical Care Medicine 2002;30(9):2032‐7. [PUBMED: 12352037] - PubMed
Grau 2011 {published data only}
-
- Grau T, Bonet A, Miñambres E, Piñeiro L, Irles JA, Robles A, et al. The effect of L‐alanyl‐L‐glutamine dipeptide supplemented total parenteral nutrition on infectious morbidity and insulin sensitivity in critically ill patients. Critical Care Medicine 2011;39(6):1263‐8. [PUBMED: 21336131] - PubMed
Griffiths 1997 {published data only}
-
- Griffiths RD, Jones C, Palmer TE. Six‐month outcome of critically ill patients given glutamine‐supplemented parenteral nutrition. Nutrition 1997;13(4):295‐302. [PUBMED: 9178278] - PubMed
Hajdú 2012 {published data only}
-
- Hajdú N, Belágyi T, Issekutz A, Bartek P, Gartner B, Oláh A. Intravenous glutamine and early nasojejunal nutrition in severe acute pancreatitis ‐a prospective randomized clinical study. Magyar Sebeszet 2012;65(2):44‐51. [PUBMED: 22512878] - PubMed
Hall 2003 {published data only}
-
- Hall JC, Dobb G, Hall J, DeSousa R, Brennan L, McCauley R. A clinical trial evaluating enteral glutamine in critically ill patients. American Journal of Clinical Nutrition 2002;75:415S.
-
- Hall JC, Dobb G, Hall J, Sousa R, Brennan L, McCauley R. A prospective randomized trial of enteral glutamine in critical illness. Intensive Care Medicine 2003;29(10):1710‐6. [PUBMED: 12923621] - PubMed
He 2004 {published data only}
-
- He XL, Ma QJ, Lu JG, Chu YK, Du XL. Effect of total parenteral nutrition (TPN) with and without glutamine dipeptide supplementation on outcome in severe acute pancreatitis (SAP). Clinical Nutrition Supplements 2004;1(1):43‐7. [DOI: 10.1016/j.clnu.2004.07.011] - DOI
Heyland 2013 {published data only}
-
- Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, et al. A randomized trial of glutamine and antioxidants in critically ill patients. New England Journal of Medicine 2013;368(16):1489‐97. [PUBMED: 23594003] - PubMed
Jiang 1999 {published data only}
-
- Jian ZM, Cao JD, Zhu XG, Zhao WX, Yu JC, Ma EL, et al. The impact of alanyl‐glutamine on clinical safety, nitrogen balance, intestinal permeability, and clinical outcome in postoperative patients: a randomized, double‐blind, controlled study of 120 patients. Journal of Parenteral and Enteral Nutrition 1999;23(5 Suppl):S62‐6. [PUBMED: 10483898] - PubMed
Karwowska 2001 {published data only}
-
- Karwowska KA, Dworacki G, Trybus M, Zeromski J, Szulc R. Influence of glutamine‐enriched parenteral nutrition on nitrogen balance and immunologic status in patients undergoing elective aortic aneurysm repair. Nutrition 2001;17(6):475‐8. [PUBMED: 11399407] - PubMed
Kumar 2007 {published data only}
-
- Kumar S, Kumar R, Sharma SB, Jain BK. Effect of oral glutamine administration on oxidative stress, morbidity and mortality in critically ill surgical patients. Indian Journal of Gastroenterology 2007;26(2):70‐3. [PUBMED: 17558069] - PubMed
Kłek 2005 {published data only}
-
- Kłek S, Kulig J, Szczepanik AM, Jedrys J, Kołodziejczyk P. The clinical value of parenteral immunonutrition in surgical patients. Acta Chirurgica Belgica 2005;105(2):175‐9. [PUBMED: 15906909] - PubMed
Lin 2002 {published data only}
-
- Lin MT, Kung SP, Yeh SL, Lin C, Lin TH, Chen KH, et al. The effect of glutamine‐supplemented total parenteral nutrition on nitrogen economy depends on severity of diseases in surgical patients. Clinical Nutrition 2002;21(3):213‐8. [PUBMED: 12127929] - PubMed
Lu 2011 {published data only}
-
- Lu CY, Shih YL, Sun LC, Chuang JF, Ma CJ, Chen FM, et al. The inflammatory modulation effect of glutamine‐enriched total parenteral nutrition in postoperative gastrointestinal cancer patients. The American Surgeon 2011;77(1):59‐64. [PUBMED: 21396307] - PubMed
Luo 2008 {published data only}
-
- Luo M, Bazargan N, Griffith DP, Estívariz CF, Leader LM, Easley KA, et al. Metabolic effects of enteral versus parenteral alanyl‐glutamine dipeptide administration in critically ill patients receiving enteral feeding: a pilot study. Clinical Nutrition 2008;27(2):297‐306. [PUBMED: 18258342] - PMC - PubMed
Mertes 2000 {published data only}
-
- Mertes N, Schulzki C, Goeters C, Winde G, Benzing S, Kuhn KS, et al. Cost containment through L‐alanyl‐L‐glutamine supplemented total parenteral nutrition after major abdominal surgery: a prospective randomized double‐blind controlled study. Clinical Nutrition 2000;19(6):395‐401. [PUBMED: 11104589] - PubMed
Morlion 1998 {published data only}
Neri 2001 {published data only}
-
- Neri A, Mariani F, Piccolomini A, Testa M, Vuolo G, Cosmo L. Glutamine‐supplemented total parenteral nutrition in major abdominal surgery. Nutrition 2001;17(11‐12):968‐9. [PUBMED: 11744350] - PubMed
O'Riordain 1994 {published data only}
Ockenga 2002 {published data only}
-
- Ockenga J, Borchert K, Rifai K, Manns MP, Bischoff SC. Effect of glutamine‐enriched total parenteral nutrition in patients with acute pancreatitis. Clinical Nutrition 2002;21(5):409‐16. [PUBMED: 12381339] - PubMed
Pattanshetti 2009 {published data only}
Peng 2004 {published data only}
-
- Peng X, Yan H, You Z, Wang P, Wang S. Clinical and protein metabolic efficacy of glutamine granules‐supplemented enteral nutrition in severely burned patients. Burns 2005;31(3):342‐6. [PUBMED: 15774291] - PubMed
-
- Peng X, Yan H, You Z, Wang P, Wang S. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns 2004;30(2):135‐9. [PUBMED: 15019120] - PubMed
-
- Peng X, Yan H, You Z, Wang P, Wang S. Glutamine granule‐supplemented enteral nutrition maintains immunological function in severely burned patients. Burns 2006;32(5):589‐93. [PUBMED: 16725264] - PubMed
Pérez‐Bárcena 2008 {published data only}
-
- Pérez‐Bárcena J, Regueiro V, Marsé P, Raurich JM, Rodríguez A, Ibáñez J, et al. Glutamine as a modulator of the immune system of critical care patients: effect on Toll‐like receptor expression. A preliminary study. Nutrition 2008;24(6):522‐7. [PUBMED: 18367379] - PubMed
Pérez‐Bárcena 2010 {published data only}
Powell‐Tuck 1999 {published data only}
Qiu 2009 {published data only}
-
- Qiu Y, Zhu X, Wang W, Xu Q, Ding Y. Nutrition support with glutamine dipeptide in patients undergoing liver transplantation. Transplantation Proceedings 2009;41(10):4232‐7. [PUBMED: 20005375] - PubMed
Sahin 2007 {published data only}
-
- Sahin H, Mercanligil SM, Inanç N, Ok E. Effects of glutamine‐enriched total parenteral nutrition on acute pancreatitis. European Journal of Clinical Nutrition 2007;61(12):1429‐34. [PUBMED: 17311061] - PubMed
Spittler 2001 {published data only}
-
- Spittler A, Sautner T, Gornikiewicz A, Manhart N, Oehler R, Bergmann M, et al. Postoperative glycyl‐glutamine infusion reduces immunosuppression: partial prevention of the surgery induced decrease in HLA‐DR expression on monocytes. Clinical Nutrition 2001;20(1):37‐42. [PUBMED: 11161542] - PubMed
Tian 2006 {published data only}
-
- Tian H, Wang KF, Wu TJ. Effect of total parenteral nutrition with supplementation of glutamine on the plasma diamine oxidase activity and D‐lactate content in patients with multiple organ dysfunction syndrome. Chinese Critical Care Medicine 2006;18(10):616‐8. - PubMed
Tjäder 2004 {published data only}
-
- Tjäder I, Rooyackers O, Forsberg AM, Vesali RF, Garlick PJ, Wernerman J. Effects on skeletal muscle of intravenous glutamine supplementation to ICU patients. Intensive Care Medicine 2004;30(2):266‐75. [PUBMED: 14722645] - PubMed
Tremel 1994 {published data only}
-
- Tremel H, Kienle B, Weilemann LS, Stehle P, Fürst P. Glutamine dipeptide‐supplemented parenteral nutrition maintains intestinal function in the critically ill. Gastroenterology 1994;107(6):1595‐601. [PUBMED: 7958669] - PubMed
Wang 2006 {published data only}
-
- Wang XY, Huang WN, Wang LY, Yang Y, Liu F, Xiao LY, et al. Effects of glutamine on plasma endotoxin and immune function in postoperative patients with laryngeal carcinoma. Chinese Journal of Clinical Nutrition 2006;14(4):222‐6.
Wernerman 2011 {published data only}
-
- Wernerman J, Kirketeig T, Andersson B, Berthelson H, Ersson A, Friberg H, et al. Scandinavian glutamine trial: a pragmatic multi‐centre randomised clinical trial of intensive care unit patients. Acta Anaesthesiologica Scandinavica 2011;55(7):812‐8. [PUBMED: 21658010] - PubMed
Wischmeyer 2001 {published data only}
-
- Wischmeyer PE, Lynch J, Liedel J, Wolfson R, Riehm J, Gottlieb L, et al. Glutamine administration reduces Gram‐negative bacteremia in severely burned patients: a prospective, randomized, double‐blind trial versus isonitrogenous control. Critical Care Medicine 2001;29(11):2075‐80. [PUBMED: 11700398] - PubMed
Yang 2008 {published data only}
-
- Yang SQ, Xu JG, Li LT, Xu J. Efect of glutamine on serum interleukin‐8 and tumor necrosis factor‐α levels in patients with severe pancreatitis. Journal of Southern Medical University 2008;28(1):129‐31. - PubMed
Yao 2007 {published data only}
-
- Yao GQ, Zhu X, Bo SN, Wang HX, Lin Y. Efect of glutamine dipeptide on nitrogen balance and immune function in patients after abdominal surgery. Chinese Journal of Clinical Nutrition 2007;15(4):223‐7.
Zhang 2007 {published data only}
-
- Zhang Z, Qin HD, Ni HB, Xu Y, Wu HR, Cheng H, et al. Effect of early enriched parenteral alanyl‐glutamine on heat shock protein 70 (HSP70) expression in critical patients. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007;19(8):481‐4. [PUBMED: 17708846] - PubMed
Zhao 2013 {published data only}
Zhou 2002 {published data only}
-
- Zhou Y, Sun Y, Jiang Z, He G, Yang N. The effects of glutamine dipeptide on the improvement of endotoxemia in severely burned patients. Zhonghua Shao Shang Za Zhi 2002;18(6):343‐5. [PUBMED: 12641984] - PubMed
Zhou 2003 {published data only}
-
- Zhou YP, Jiang ZM, Sun YH, Wang XR, Ma EL, Wilmore D. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: a randomized, double‐blind, controlled clinical trial. Journal of Parenteral and Enteral Nutrition 2003;27(4):241‐5. [PUBMED: 12903886 ] - PubMed
Zhou 2004 {published data only}
-
- Zhou YP, Jiang ZM, Sun YH, He GZ, Shu H. The effects of supplemental glutamine dipeptide on gut integrity and clinical outcome after major escharectomy in severe burns: a randomized, double‐blind, controlled clinical trial. Clinical Nutrition Supplements 2004;1(1):55‐60. [DOI: 10.1016/j.clnu.2004.07.012] - DOI
Zhu 2000 {published data only}
-
- Zhu M, Tang D, Zhao X, Cao J, Wei J, Chen Y, et al. Impact of glutamine of gut permeability and clinical prognosis on the aging patients undergoing gastric‐intestinal operation. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2000;22(5):425‐7. [PUBMED: 12903420] - PubMed
References to studies excluded from this review
Boelens 2002 {published data only}
-
- Boelens PG, Houdijk AP, Fonk JC, Nijveldt RJ, Ferwerda CC, Blomberg‐Van Der Flier BM, et al. Glutamine‐enriched enteral nutrition increases HLA‐DR expression on monocytes of trauma patients. Journal of Nutrition 2002;132(9):2580‐6. [PUBMED: 12221212] - PubMed
Boelens 2003 {published data only}
-
- Boelens PG, Houdijk AP, Thouars HN, Teerlink T, Engeland MI, Haarman HJ, et al. Plasma taurine concentrations increase after enteral glutamine supplementation in trauma patients and stressed rats. American Journal of Clinical Nutrition 2003;77(1):250‐6. [PUBMED: 12499349] - PubMed
Brantley 2000 {published data only}
-
- Brantley S, Pierce J. Effects of enteral glutamine on trauma patients. Nutrition in Clinical Practice 2000;15:S13. [http://ncp.sagepub.com/content/15/3/S11.full.pdf+html]
Duska 2008a {published data only}
-
- Duska F, Fric M, Waldauf P, Pazout J, Andel M, Mokrejs P, et al. Frequent intravenous pulses of growth hormone together with glutamine supplementation in prolonged critical illness after multiple trauma: effects on nitrogen balance, insulin resistance, and substrate oxidation. Critical Care Medicine 2008;36(6):1707‐13. [PUBMED: 18496372] - PubMed
Duska 2008b {published data only}
-
- Duska F, Fric M, Pazout J, Waldauf P, Tůma P, Pachl J. Frequent intravenous pulses of growth hormone together with alanylglutamine supplementation in prolonged critical illness after multiple trauma: effects on glucose control, plasma IGF‐I and glutamine. Growth Hormone & IGF Research 2008;18(1):82‐7. [PUBMED: 17709266] - PubMed
Griffiths 2002 {published data only}
-
- Griffiths RD, Allen KD, Andrews FJ, Jones C. Infection, multiple organ failure, and survival in the intensive care unit: influence of glutamine‐supplemented parenteral nutrition on acquired infection. Nutrition 2002;18(7‐8):546‐52. [PUBMED: 12093428] - PubMed
Grigoryev 2010 {published data only}
-
- Grigoryev EG. Therapy of compartmentalization of the proinflammatory and anti‐inflammatory cytokines in sepsis. Critical Care 2010;14 Suppl 1:P566. [DOI: 10.1186/cc8798] - DOI
Gurnani 2012 {published data only}
-
- Gurnani A, Agarwal A. Immunonutrition and correlation with biomarkers in critically ill an Indian perspective. Clinical Nutrition, Supplement 2012;7(1):144.
Heyland 2007 {published data only}
-
- Heyland DK, Dhaliwalm R, Day A, Drover J, Cote H, Wischmeyer P. Optimizing the dose of glutamine dipeptides and antioxidants in critically ill patients: a phase I dose‐finding study. Journal of Parenteral and Enteral Nutrition 2007;31(2):109‐18. [PUBMED: 17308251] - PubMed
Houdijk 1998 {published data only}
-
- Houdijk AP, Rijnsburger ER, Jansen J, Wesdorp RI, Weiss JK, McCamish MA, et al. Randomised trial of glutamine‐enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet 1998;352(9130):772‐6. [PUBMED: 9737282] - PubMed
Jiang 2007 {published data only}
-
- Jiang H, Li B, Yan LN, Lu SC, Wen TF, Zhao JC, et al. Effect of Intravenous glutamine‐dipeptide fortified enteral nutrition on clinical outcomes in patients after liver transplantation: A prospective randomized controlled study. Chinese Journal of Clinical Nutrition 2007;15(1):21‐5.
Jones 1999 {published data only}
-
- Jones C, Palmer TE, Griffiths RD. Randomized clinical outcome study of critically ill patients given glutamine‐supplemented enteral nutrition. Nutrition 1999;15(2):108‐15. [PUBMED: 9990574] - PubMed
Juan 2012 {published data only}
-
- Juan M, Mesejo A, Serrano A, Corcobado C, Bueno A, Yuste L, et al. Comparison of three enteral formulas to glycemic and infectious control in critically ill patients under mechanical ventilation: High‐protein, high‐protein disease‐specific and disease‐specific diet supplemented with glutamine. Intensive Care Medicine 2012;38:S122.
Koksal 2011 {published data only}
-
- Koksal G, Karaoren G, Akarcay H, Karabulut E, Tunali Y, Vehid S, et al. Comparison of the effects of intravenous, enteral and enteral + intravenous supply of glutamine on malnutrition in sepsis. 31st International Symposium on Intensive Care and Emergency Medicine; 2011; Brussels, Belgium. Critical Care 15. S137.
Liu 2012 {published data only}
-
- Liu H, Ling W, Shen ZY, Jin X, Cao H. Clinical application of immune‐enhanced enteral nutrition in patients with advanced gastric cancer after total gastrectomy. Journal of Digestive Diseases 2012;13(8):401‐6. [PUBMED: 22788925] - PubMed
Mangano 1993 {published data only}
-
- Mangano A, Panza N, Paganini N, Croce G, Cassone E, Trevisan M, et al. Effects of parenteral glutamine supplementation on the nutritional status of patients with radical cystectomy in neoplasia. Acta Urologica Italica 1993;7 Suppl 2:119‐20. [EMBASE: 1994073349]
McQuiggan 2008 {published data only}
-
- McQuiggan M, Kozar R, Sailors RM, Ahn C, McKinley B, Moore F. Enteral glutamine during active shock resuscitation is safe and enhances tolerance of enteral feeding. Journal of Parenteral and Enteral Nutrition 2008;32(1):28‐35. [PUBMED: 18165444] - PubMed
Nitta 2001 {published data only}
-
- Nitta H, Ikeda K, Aoki K. Effects of perioperative great amount of glutamine supplementation by enteral route on amino acids metabolism. Journal of Parenteral and Enteral Nutrition 2001;25:S22. [http://pen.sagepub.com/content/25/1/S1.full.pdf+html]
Ozgultekin 2008 {published data only}
-
- Ozgultekin A, Turan G, Durmus Y, Dincer E, Akgun N. Comparison of the efficacy of parenteral glutamine and branched‐chain amino acid solutions given as extra supplements in parallel to the enteral nutrition in head trauma. e‐SPEN, the European e‐Journal of Clinical Nutrition and Metabolism 2008;3(5):e211‐6. [DOI: 10.1016/j.eclnm.2008.05.006] - DOI
Palmese 2006 {published data only}
-
- Palmese S, Odierna I, Scarano D, Scibili AC, Natale A, Pezza M. Early enteral nutrition enriched with FOS and intravenous glutamine supplementation in intensive care unit patients. Nutritional Therapy Metabolism 2006;24(3):140‐6. [DOI: 10.5301/NTM.2012.9433] - DOI
Schulman 2005 {published data only}
-
- Schulman AS, Willcutts KF, Claridge JA, Evans HL, Radigan AE, O'Donnell KB, et al. Does the addition of glutamine to enteral feeds affect patient mortality?. Critical Care Medicine 2005;33(11):2501‐6. [PUBMED: 16276173] - PubMed
Xiangdong 2012 {published data only}
-
- Xiangdong G, Xiaoyue L. The impact of glutamine on organ function and mortality of patients with severe sepsis. Intensive Care Medicine 2012;38:S31.
Xue 2008 {published data only}
Ziegler 2004 {published data only}
-
- Ziegler TR, Fernandez‐Estivariz C, Griffith DP, Szeszycki EE, Bazargan N, Luo M, et al. Parenteral nutrition supplemented with alanyl glutamine dipeptide decreases infectious morbidity and improves organ function in critically ill post‐operative patients: results of a double‐blind,randomized,controlled pilot study. Journal of Parenteral and Enteral Nutrition 2004;28:S11‐2.
Ziegler 2012 {published data only}
-
- Ziegler T, May A. Glutamine dipeptide‐supplemented parenteral nutrition in surgical ICU patients: Results of an American randomized, double‐blind, multicenter trial. Clinical Nutrition, Supplement 2012;7(1):265.
References to studies awaiting assessment
Hallay 2002 {published data only}
-
- Hallay J, Kovács G, Kiss Sz S, Farkas M, Lakos G, Sipka S, et al. Changes in the nutritional state and immune‐serological parameters of esophagectomized patients fed jejunaly with glutamine‐poor and glutamine‐rich nutriments. Hepatogastroenterology 2002;49(48):1555‐9. [PUBMED: 12397734] - PubMed
References to ongoing studies
Al Balushi 2010 {published data only}
Pérez‐Barcena 2010 {published data only}
-
- Effectiveness of Dipeptide N (2)‐L‐Alanyl‐L‐Glutamine in Trauma ICU Patients: Pilot, Prospective, Randomized and Double Blind Study. Ongoing study October 2010.
Rastmanesh 2010 {published data only}
-
- Iranian Intensive Care Unit (ICU) Glutamine Study. Ongoing study October 2010.
Talukdar 2012 {published data only}
-
- Efficacy of Enteral Glutamine Supplementation in Patients With Predicted Severe Acute Pancreatitis‐ A Double‐Blinded Randomized Controlled Trial. Ongoing study January 2012.
Wischmeyer 2010 {published data only}
Ziegler 2013 {published data only}
-
- A Randomized Controlled Trial of Glutamine Dipeptide in Severe Trauma (GLND Trauma). Ongoing study February 2013.
Additional references
Avenell 2006
-
- Avenell A. Glutamine in critical care: current evidence from systematic reviews. Proceedings of the Nutrition Society 2006;65(3):236‐41. [PUBMED: 16923308] - PubMed
Avenell 2009
-
- Avenell A. Hot topics in parenteral nutrition. Current evidence and ongoing trials on the use of glutamine in critically‐ill patients and patients undergoing surgery. Proceedings of the Nutrition Society 2009;68(3):261‐8. [PUBMED: 19490739] - PubMed
Bakalar 2006
-
- Bakalar B, Duska F, Pachl J, Fric M, Otahal M, Pazout J, et al. Parenterally administered dipeptide alanyl‐glutamine prevents worsening of insulin sensitivity in multiple‐trauma patients. Critical Care Medicine 2006;34(2):381‐6. [PUBMED: 16424718] - PubMed
Bollhalder 2013
-
- Bollhalder L, Pfeil AM, Tomonaga Y, Schwenkglenks M. A systematic literature review and meta‐analysis of randomized clinical trials of parenteral glutamine supplementation. Clinical Nutrition 2013;32(2):213‐23. [PUBMED: 23196117] - PubMed
Bongers 2007
-
- Bongers T, Griffiths RD, McArdle A. Exogenous glutamine: the clinical evidence. Critical Care Medicine 2007;35(9 Suppl):S545‐52. [PUBMED: 17713407] - PubMed
Clinical Evaluation Research Unit 2013
-
- Clinical Evaluation Research Unit. Canadian Clinical Practice Guidelines: critical care nutrition. Available from www.criticalcarenutrition.com accessed on 1st July 2013.
Déchelotte 2006
-
- Déchelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coëffier M, Hecketsweiler B, et al. L‐alanyl‐L‐glutamine dipeptide‐supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double‐blind, multicenter study. Critical Care Medicine 2006;34(3):598‐604. [PUBMED: 16505644] - PubMed
Gamrin 1996
-
- Gamrin L, Essén P, Forsberg AM, Hultman E, Wernerman J. A descriptive study of skeletal muscle metabolism in critically ill patients: free amino acids, energy‐rich phosphates, protein, nucleic acids, fat, water, and electrolytes. Critical Care Medicine 1996;24(4):575‐83. [PUBMED: 8612406 ] - PubMed
Guyatt 2008
Hammarqvist 1989
-
- Hammarqvist F, Wernerman J, Ali R, Decken A, Vinnars E. Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares free glutamine in muscle, counteracts the fall in muscle protein synthesis, and improves nitrogen balance. Annals of Surgery 1989;209(4):455‐61. [PUBMED: 2494960] - PMC - PubMed
Handbook 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org accessed on 1st July 2013.
Heyland 2006
-
- Heyland DK, Dhaliwal R, Day AG, Muscedere J, Drover J, Suchner U, et al. REducing Deaths due to OXidative Stress (The REDOXS Study): rationale and study design for a randomized trial of glutamine and antioxidant supplementation in critically‐ill patients. Proceedings of the Nutrition Society 2006;65(3):250‐63. [PUBMED: 16923310] - PubMed
Heyland 2013 [pers comm]
-
- Heyland D. RE: Help on the article "A randomized trial of glutamine and antioxidants in critically ill patients". E‐mail to: KM Tao 27 June 2013. - PubMed
Hozo 2005
Jones 1993
-
- Jones C, Hussey R, Griffiths RD. A tool to measure the change in health status of selected adult patients before and after intensive care. Clinical Intensive Care 1993;4(4):160‐5. [PUBMED: 10146456] - PubMed
Kim 2013
-
- Kim M, Wischmeyer PE. Glutamine. World Review of Nutrition and Dietetics 2013;105:90‐6. [PUBMED: 23075590] - PubMed
Koretz 2007a
-
- Koretz RL, Avenell A, Lipman TO, Braunschweig CL, Milne AC. Does enteral nutrition affect clinical outcome? A systematic review of the randomized trials. American Journal of Gastroenterology 2007;102(2):412‐29. [PUBMED: 17311654] - PubMed
Koretz 2007b
-
- Koretz RL. Do data support nutrition support? Part I: intravenous nutrition. Journal of the American Dietetic Association 2007;107(6):988‐96. [PUBMED: 17524720] - PubMed
Koretz 2007c
-
- Koretz RL. Do data support nutrition support? Part II. enteral artificial nutrition. Journal of the American Dietetic Association 2007;107(8):1374‐80. [PUBMED: 17659905] - PubMed
Kreymann 2006
-
- Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN Guidelines on Enteral Nutrition: Intensive care. Clinical Nutrition 2006;25(2):210‐23. [PUBMED: 16697087 ] - PubMed
Melis 2004
-
- Melis GC, ter Wengel N, Boelens PG, Leeuwen PA. Glutamine: recent developments in research on the clinical significance of glutamine. Current Opinion in Clinical Nutrition and Metabolic Care 2004;7(1):59‐70. [PUBMED: 15090905] - PubMed
Mizock 2010
-
- Mizock BA. Immunonutrition and critical illness: an update. Nutrition 2010;26(7‐8):701‐7. [PUBMED: 20381315] - PubMed
Novak 2002
-
- Novak F, Heyland DK, Avenell A, Drover JW, Su X. Glutamine supplementation in serious illness: a systematic review of the evidence. Critical Care Medicine 2002;30(9):2022‐9. [PUBMED: 12352035] - PubMed
O'Leary 1996
-
- O'Leary MJ, Coakley JH. Nutrition and immunonutrition. British Journal of Anaesthesia 1996;77(1):118‐27. [PUBMED: 8703621] - PubMed
O'Riordain 1996
-
- O'Riordain MG, Beaux A, Fearon KC. Effect of glutamine on immune function in the surgical patient. Nutrition 1996;12(11‐12 Suppl):S82‐4. [PUBMED: 8974126] - PubMed
Ogle 1994
-
- Ogle CK, Ogle JD, Mao JX, Simon J, Noel JG, Li BG, et al. Effect of glutamine on phagocytosis and bacterial killing by normal and pediatric burn patient neutrophils. Journal of Parenteral and Enteral Nutrition 1994;18(2):128‐33. [PUBMED: 8201747] - PubMed
Parry‐Billings 1992
-
- Parry‐Billings M, Baigrie RJ, Lamont PM, Morris PJ, Newsholme EA. Effects of major and minor surgery on plasma glutamine and cytokine levels. Archives of Surgery 1992;127(10):1237‐40. [PUBMED: 1358047] - PubMed
RevMan 5.2 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roth 2008
-
- Roth E. Nonnutritive effects of glutamine. The Journal of Nutrition 2008;138(10):2025S‐31S. [PUBMED: 18806119] - PubMed
Salloum 1991
Singer 2009
-
- Singer P, Berger MM, Berghe G, Biolo G, Calder P, Forbes A, et al. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clinical Nutrition 2009;28(4):387‐400. [PUBMED: 19505748] - PubMed
Tjader 2007
-
- Tjader I, Berg A, Wernerman J. Exogenous glutamine‐compensating a shortage?. Critical Care Medicine 2007;35(9 Suppl):S553‐6. [PUBMED: 17713408] - PubMed
Tschoeke 2007
-
- Tschoeke SK, Ertel W. Immunoparalysis after multiple trauma. Injury 2007;38(12):1346‐57. [PUBMED: 18048039] - PubMed
Vinnars 1975
Wang 2010
-
- Wang Y, Jiang ZM, Nolan MT, Jiang H, Han HR, Yu K, et al. The impact of glutamine dipeptide‐supplemented parenteral nutrition on outcomes of surgical patients: a meta‐analysis of randomized clinical trials. Journal of Parenteral and Enteral Nutrition 2010;34(5):521‐9. [PUBMED: 20852180] - PubMed
Yue 2013
-
- Yue C, Tian W, Wang W, Huang Q, Zhao R, Zhao Y, et al. The impact of perioperative glutamine‐supplemented parenteral nutrition on outcomes of patients undergoing abdominal surgery: a meta‐analysis of randomized clinical trials. The American Surgeon 2013;79(5):506‐13. [PUBMED: 23635587] - PubMed
Zheng 2006
Ziegler 2005
-
- Ziegler TR, Ogden LG, Singleton KD, Luo M, Fernandez‐Estivariz C, Griffith DP, et al. Parenteral glutamine increases serum heat shock protein 70 in critically ill patients. Intensive Care Medicine 2005;31(8):1079‐86. [PUBMED: 15973519] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

