Immunological markers of Plasmodium vivax exposure and immunity: a systematic review and meta-analysis
- PMID: 25199532
- PMCID: PMC4172944
- DOI: 10.1186/s12916-014-0150-1
Immunological markers of Plasmodium vivax exposure and immunity: a systematic review and meta-analysis
Abstract
Background: Identifying Plasmodium vivax antigen-specific antibodies associated with P. vivax infection and protective immunity is key to the development of serosurveillance tools and vaccines for malaria. Antibody targets of P. vivax can be identified by seroepidemiological studies of individuals living in P. vivax-endemic areas, and is an important strategy given the limited ability to culture P. vivax in vitro. There have been numerous studies investigating the association between P. vivax antibody responses and P. vivax infection, but there has been no standardization of results to enable comparisons across populations.
Methods: We performed a systematic review with meta-analysis of population-based, cross-sectional, case-control, and cohort studies of individuals living in P. vivax-endemic areas. We searched 6 databases and identified 18 studies that met predefined inclusion and quality criteria, and examined the association between antibody responses to P. vivax antigens and P. vivax malaria.
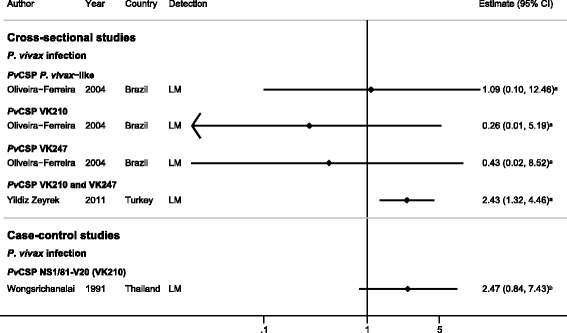
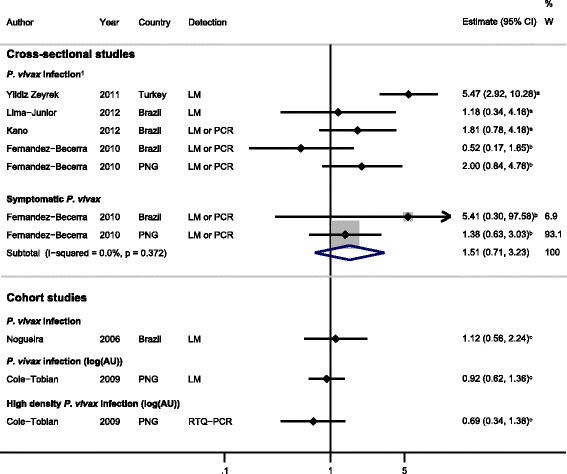
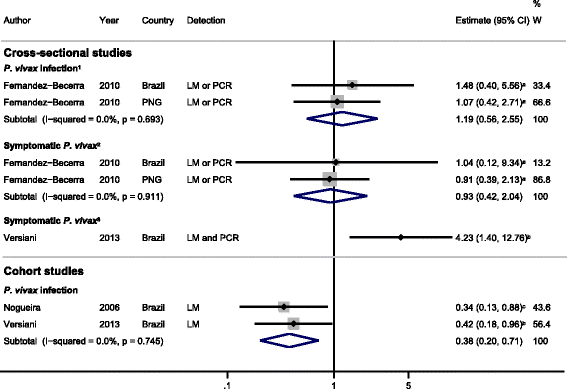
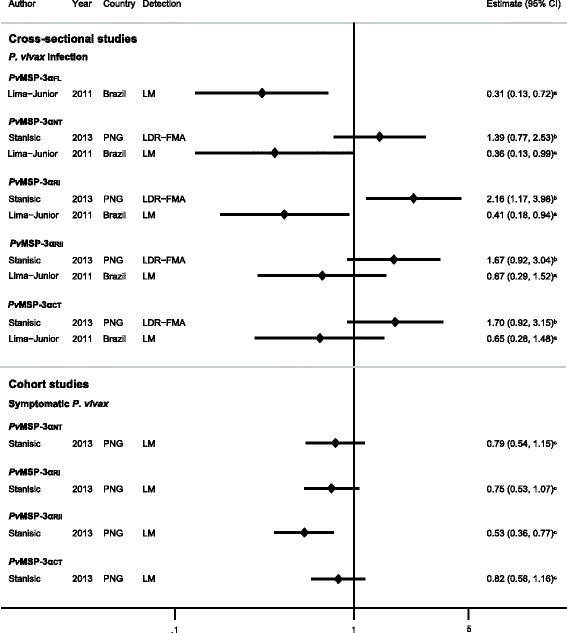
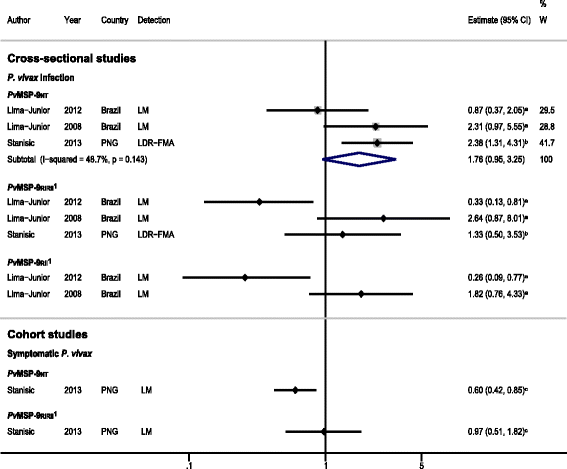
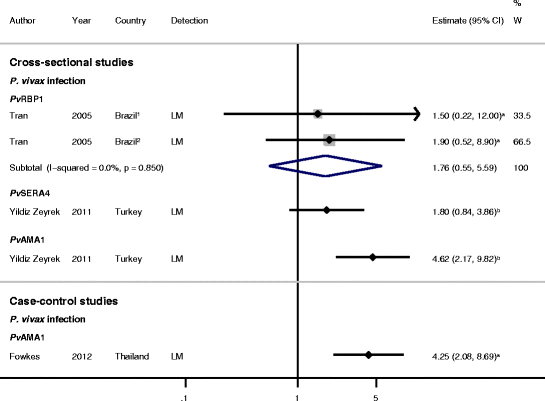
Results: The majority of studies were published in South America (all from Brazil) and the rest from geographically diverse areas in the Asia-Pacific region. Considerable heterogeneity in estimates was observed, but IgG responses to PvCSP, PvMSP-119, PvMSP-9RIRII, and PvAMA1 were associated with increased odds of P. vivax infection in geographically diverse populations. Potential sources of heterogeneity included study design, different transmission intensities and transmigrant populations. Protective associations were observed for antibodies to PvMSP-119, PvMSP-1NT, PvMSP-3α and PvMSP-9NT antigens, but only in single geographical locations.
Conclusions: This systematic review revealed several antigen-specific antibodies that were associated with active infection and protective immunity, which may be useful biomarkers. However, more studies are needed on additional antigens, particularly cohort studies to increase the body of evidence for protective immunity. More studies representing diverse geographical regions encompassing varying P. vivax endemicities are needed to validate the generalizability of the findings and to provide a solid evidence base for the use of P. vivax antigens in vaccines and serosurveillance tools.
Figures


References
-
- Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS Negl Trop Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. - DOI - PMC - PubMed
-
- WHO: Malaria Vaccine Rainbow Tables. 2014. http://www.who.int/vaccine_research/links/Rainbow/en/index.html.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

