Local CRH signaling promotes synaptogenesis and circuit integration of adult-born neurons
- PMID: 25199688
- PMCID: PMC4182170
- DOI: 10.1016/j.devcel.2014.07.001
Local CRH signaling promotes synaptogenesis and circuit integration of adult-born neurons
Abstract
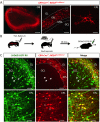
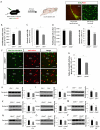
Neural activity either enhances or impairs de novo synaptogenesis and circuit integration of neurons, but how this activity is mechanistically relayed in the adult brain is largely unknown. Neuropeptide-expressing interneurons are widespread throughout the brain and are key candidates for conveying neural activity downstream via neuromodulatory pathways that are distinct from classical neurotransmission. With the goal of identifying signaling mechanisms that underlie neuronal circuit integration in the adult brain, we have virally traced local corticotropin-releasing hormone (CRH)-expressing inhibitory interneurons with extensive presynaptic inputs onto new neurons that are continuously integrated into the adult rodent olfactory bulb. Local CRH signaling onto adult-born neurons promotes and/or stabilizes chemical synapses in the olfactory bulb, revealing a neuromodulatory mechanism for continued circuit plasticity, synapse formation, and integration of new neurons in the adult brain.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Sculpting circuits: CRH interneurons modulate neuronal integration.Dev Cell. 2014 Sep 29;30(6):639-40. doi: 10.1016/j.devcel.2014.09.006. Dev Cell. 2014. PMID: 25268169
Similar articles
-
POU6f1 Mediates Neuropeptide-Dependent Plasticity in the Adult Brain.J Neurosci. 2018 Feb 7;38(6):1443-1461. doi: 10.1523/JNEUROSCI.1641-17.2017. Epub 2018 Jan 5. J Neurosci. 2018. PMID: 29305536 Free PMC article.
-
Local corticotropin releasing hormone (CRH) signals to its receptor CRHR1 during postnatal development of the mouse olfactory bulb.Brain Struct Funct. 2016 Jan;221(1):1-20. doi: 10.1007/s00429-014-0888-4. Epub 2014 Sep 16. Brain Struct Funct. 2016. PMID: 25224546 Free PMC article.
-
Sculpting circuits: CRH interneurons modulate neuronal integration.Dev Cell. 2014 Sep 29;30(6):639-40. doi: 10.1016/j.devcel.2014.09.006. Dev Cell. 2014. PMID: 25268169
-
Inhibitory interneurons in the olfactory bulb: from development to function.Neuroscientist. 2004 Aug;10(4):292-303. doi: 10.1177/1073858404263460. Neuroscientist. 2004. PMID: 15271257 Review.
-
Watching synaptogenesis in the adult brain.Annu Rev Neurosci. 2010;33:131-49. doi: 10.1146/annurev-neuro-060909-153252. Annu Rev Neurosci. 2010. PMID: 20572770 Review.
Cited by
-
Experiences Shape Hippocampal Neuron Morphology and the Local Levels of CRHR1 and OTR.Cell Mol Neurobiol. 2023 Jul;43(5):2129-2147. doi: 10.1007/s10571-022-01292-7. Epub 2022 Oct 14. Cell Mol Neurobiol. 2023. PMID: 36239833 Free PMC article.
-
Signaling mechanisms underlying activity-dependent integration of adult-born neurons in the mouse olfactory bulb.Genesis. 2024 Apr;62(2):e23595. doi: 10.1002/dvg.23595. Genesis. 2024. PMID: 38553878 Free PMC article. Review.
-
Gut neuroendocrine signaling regulates synaptic assembly in C. elegans.EMBO Rep. 2022 Aug 3;23(8):e53267. doi: 10.15252/embr.202153267. Epub 2022 Jun 24. EMBO Rep. 2022. PMID: 35748387 Free PMC article.
-
Latrotoxin-Induced Neuromuscular Junction Degeneration Reveals Urocortin 2 as a Critical Contributor to Motor Axon Terminal Regeneration.Int J Mol Sci. 2022 Jan 21;23(3):1186. doi: 10.3390/ijms23031186. Int J Mol Sci. 2022. PMID: 35163106 Free PMC article.
-
Sensory experience shapes the integration of adult-born neurons into the olfactory bulb.J Nat Sci. 2017 Aug;3(8):e422. J Nat Sci. 2017. PMID: 28884145 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

