In vitro testing for diagnosis of idiosyncratic adverse drug reactions: Implications for pathophysiology
- PMID: 25199801
- PMCID: PMC4594732
- DOI: 10.1111/bcp.12505
In vitro testing for diagnosis of idiosyncratic adverse drug reactions: Implications for pathophysiology
Abstract
Idiosyncratic drug reactions (IDRs) represent a major health problem, as they are unpredictable, often severe and can be life threatening. The low incidence of IDRs makes their detection during drug development stages very difficult causing many post-marketing drug withdrawals and black box warnings. The fact that IDRs are always not predictable based on the drug's known pharmacology and have no clear dose-effect relationship with the culprit drug renders diagnosis of IDRs very challenging, if not impossible, without the aid of a reliable diagnostic test. The drug provocation test (DPT) is considered the gold standard for diagnosis of IDRs but it is not always safe to perform on patients. In vitro tests have the advantage of bearing no potential harm to patients. However, available in vitro tests are not commonly used clinically because of lack of validation and their complex and expensive procedures. This review discusses the current role of in vitro diagnostic testing for diagnosis of IDRs and gives a brief account of their technical and mechanistic aspects. Advantages, disadvantages and major challenges that prevent these tests from becoming mainstream diagnostic tools are also discussed here.
Keywords: adverse drug events; adverse drug reactions; drug allergy; idiosyncratic drug reactions; in vitro diagnosis.
© 2014 The British Pharmacological Society.
Figures
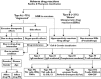
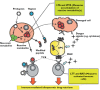
 ); reactive parent drug (
); reactive parent drug ( ); reactive metabolite (
); reactive metabolite ( )
)References
-
- Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, Brockow K, Pichler WJ, Demoly P. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003;58:854–863. - PubMed
-
- Elzagallaai AA, Knowles SR, Rieder MJ, Bend JR, Shear NH, Koren G. In vitro tests for the diagnosis of anticonvulsant hypersensitivity syndrome (AHS): a systematic review. Mol Diagn Ther. 2009;13:313–330. - PubMed
-
- Mayorga C, Sanz ML, Gamboa P, Garcia-Aviles MC, Fernandez J, Torres MJ. In vitro methods for diagnosing nonimmediate hypersensitivity reactions to drugs. J Investig Allergol Clin Immunol. 2013;23:213–225. ; quiz precedeing 225. - PubMed
-
- Rawlins M, Thompson J. Pathogenesis of adverse drug reactions. In: Davies D, editor. Textbook of Adverse Drug Reactions. Oxford: Oxford University Press; 1977. p. 10.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

