Regulation of proximal T cell receptor signaling and tolerance induction by deubiquitinase Usp9X
- PMID: 25200027
- PMCID: PMC4172213
- DOI: 10.1084/jem.20140860
Regulation of proximal T cell receptor signaling and tolerance induction by deubiquitinase Usp9X
Abstract
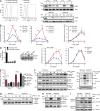
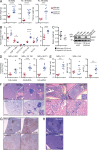
The T cell hyperproliferation and autoimmune phenotypes that manifest in mice lacking E3 ubiquitin ligases such as Cbl, ITCH, or GRAIL highlight the importance of ubiquitination for the maintenance of peripheral T cell tolerance. Less is known, however, about the deubiquitinating enzymes that regulate T cell proliferation and effector function. Here, we define a cell intrinsic role for the deubiquitinase Usp9X during proximal TCR signaling. Usp9X-deficient T cells were hypoproliferative, yet mice with T cell-specific Usp9x deletion had elevated numbers of antigen-experienced T cells and expanded PD-1 and OX40-expressing populations consistent with immune hyperactivity. Aged Usp9x KO mice developed lupus-like autoimmunity and lymphoproliferative disease, indicating that ubiquitin ligases and deubiquitinases maintain the delicate balance between effective immunity and self-tolerance.
© 2014 Naik et al.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

