Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer
- PMID: 25200057
- PMCID: PMC4862832
- DOI: 10.1161/CIRCULATIONAHA.113.007917
Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer
Abstract
Background: Despite the promise shown by stem cells for restoration of cardiac function after myocardial infarction, the poor survival of transplanted cells has been a major issue. Hypoxia-inducible factor-1 (HIF1) is a transcription factor that mediates adaptive responses to ischemia. Here, we hypothesize that codelivery of cardiac progenitor cells (CPCs) with a nonviral minicircle plasmid carrying HIF1 (MC-HIF1) into the ischemic myocardium can improve the survival of transplanted CPCs.
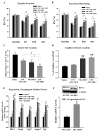
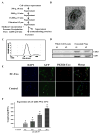
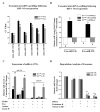
Methods and results: After myocardial infarction, CPCs were codelivered intramyocardially into adult NOD/SCID mice with saline, MC-green fluorescent protein, or MC-HIF1 versus MC-HIF1 alone (n=10 per group). Bioluminescence imaging demonstrated better survival when CPCs were codelivered with MC-HIF1. Importantly, echocardiography showed mice injected with CPCs+MC-HIF1 had the highest ejection fraction 6 weeks after myocardial infarction (57.1±2.6%; P=0.002) followed by MC-HIF1 alone (48.5±2.6%; P=0.04), with no significant protection for CPCs+MC-green fluorescent protein (44.8±3.3%; P=NS) when compared with saline control (38.7±3.2%). In vitro mechanistic studies confirmed that cardiac endothelial cells produced exosomes that were actively internalized by recipient CPCs. Exosomes purified from endothelial cells overexpressing HIF1 had higher contents of miR-126 and miR-210. These microRNAs activated prosurvival kinases and induced a glycolytic switch in recipient CPCs, giving them increased tolerance when subjected to in vitro hypoxic stress. Inhibiting both of these miRs blocked the protective effects of the exosomes.
Conclusions: In summary, HIF1 can be used to modulate the host microenvironment for improving survival of transplanted cells. The exosomal transfer of miRs from host cells to transplanted cells represents a unique mechanism that can be potentially targeted for improving survival of transplanted cells.
Keywords: exosomes; genetic therapy; hypoxia-inducible factor-1; microRNAs; stem cells.
© 2014 American Heart Association, Inc.
Figures



Comment in
-
Letter by Li and Hong regarding article, "Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer".Circulation. 2015 Mar 24;131(12):e384. doi: 10.1161/CIRCULATIONAHA.114.013645. Circulation. 2015. PMID: 25802263 No abstract available.
-
Response to letter regarding article, "Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer".Circulation. 2015 Mar 24;131(12):e385. doi: 10.1161/CIRCULATIONAHA.114.014467. Circulation. 2015. PMID: 25802264 No abstract available.
References
-
- Hausenloy DJ, Erik Botker H, Condorelli G, Ferdinandy P, Garcia-Dorado D, Heusch G, Lecour S, van Laake LW, Madonna R, Ruiz-Meana M, Schulz R, Sluijter JP, Yellon DM, Ovize M. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2013;98:7–27. - PubMed
-
- Liu J, Narsinh KH, Lan F, Wang L, Nguyen PK, Hu S, Lee A, Han L, Gong Y, Huang M, Nag D, Rosenberg J, Chouldechova A, Robbins RC, Wu JC. Early stem cell engraftment predicts late cardiac functional recovery: preclinical insights from molecular imaging. Circ Cardiovasc Imaging. 2012;5:481–90. - PMC - PubMed
-
- Ong SG, Hausenloy DJ. Hypoxia-inducible factor as a therapeutic target for cardioprotection. Pharmacol Ther. 2012;136:69–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

