Twist1 expression induced by sunitinib accelerates tumor cell vasculogenic mimicry by increasing the population of CD133+ cells in triple-negative breast cancer
- PMID: 25200065
- PMCID: PMC4168051
- DOI: 10.1186/1476-4598-13-207
Twist1 expression induced by sunitinib accelerates tumor cell vasculogenic mimicry by increasing the population of CD133+ cells in triple-negative breast cancer
Abstract
Background: Hypoxia induced by antiangiogenic agents is linked to the generation of cancer stem cells (CSCs) and treatment failure through unknown mechanisms. The generation of endothelial cell-independent microcirculation in malignant tumors is defined as tumor cell vasculogenic mimicry (VM). In the present study, we analyzed the effects of an antiangiogenic agent on VM in triple-negative breast cancer (TNBC).
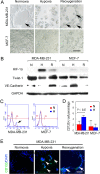
Methods: Microcirculation patterns were detected in patients with TNBC and non-TNBC. Tientsin Albino 2 (TA2) mice engrafted with mouse TNBC cells and nude mice engrafted with human breast cancer cell lines with TNBC or non-TNBC phenotypes were administered sunitinib and analyzed to determine tumor progression, survival, microcirculation, and oxygen concentration. Further, we evaluated the effects of hypoxia induced with CoCl2 and the expression levels of the transcription factor Twist1, in the presence or absence of a Twist siRNA, on the population of CD133(+) cells and VM in TNBC and non-TNBC cells.
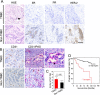
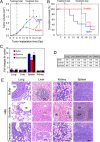
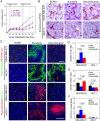
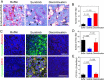
Results: VM was detected in 35.8 and 17.8% of patients with TNBC or with non-TNBC, respectively. The growth of tumors in TNBC and non-TNBC-bearing mice was inhibited by sunitinib. The tumors in TA2 mice engrafted with mouse TNBCs and in mice engrafted a human TNBC cell line (MDA-MB-231) regrew after terminating sunitinib administration. However, this effect was not observed in mice engrafted with a non-TNBC tumor cell line. Tumor metastases in sunitinib-treated TA2 mice was accelerated, and the survival of these mice decreased when sunitinib was withdrawn. VM was the major component of the microcirculation in sunitinib-treated mice with TNBC tumors, and the population of CD133+ cells increased in hypoxic areas. Hypoxia also induced MDA-MB-231 cells to express Twist1, and CD133(+) cells present in the MDA-MB-231 cell population induced VM after reoxygenation. Moreover, hypoxia did not induce MDA-MB-231 cells transfected with an sh-Twist1 siRNA cell to form VM and generate CD133(+) cells. Conversely, hypoxia induced MCF-7 cells transfected with Twist to form VM and generate CD133+ cells.
Conclusions: Sunitinib induced hypoxia in TNBCs, and Twist1 expression induced by hypoxia accelerated VM by increasing population of CD133(+) cells. VM was responsible for the regrowth of TNBCs sunitinib administration was terminated.
Figures

References
-
- von Minckwitz G, Untch M, Nuesch E, Loibl S, Kaufmann M, Kummel S, Fasching PA, Eiermann W, Blohmer JU, Costa SD, Mehta K, Hilfrich J, Jackisch C, Gerber B, du Bois A, Huober J, Hanusch C, Konecny G, Fett W, Stickeler E, Harbeck N, Müller V, Jüni P. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat. 2011;125:145–156. doi: 10.1007/s10549-010-1228-x. - DOI - PubMed
-
- Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825–2831. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

