Direct binding of the Alu binding protein dimer SRP9/14 to 40S ribosomal subunits promotes stress granule formation and is regulated by Alu RNA
- PMID: 25200073
- PMCID: PMC4176187
- DOI: 10.1093/nar/gku822
Direct binding of the Alu binding protein dimer SRP9/14 to 40S ribosomal subunits promotes stress granule formation and is regulated by Alu RNA
Abstract
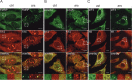
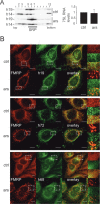
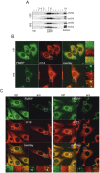
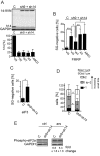
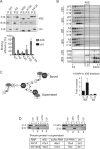
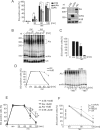
Stress granules (SGs) are formed in response to stress, contain mRNAs, 40S ribosomal subunits, initiation factors, RNA-binding and signaling proteins, and promote cell survival. Our study describes a novel function of the protein heterodimer SRP9/14 and Alu RNA in SG formation and disassembly. In human cells, SRP9/14 exists assembled into SRP, bound to Alu RNA and as a free protein. SRP9/14, but not SRP, localizes to SGs following arsenite or hippuristanol treatment. Depletion of the protein decreases SG size and the number of SG-positive cells. Localization and function of SRP9/14 in SGs depend primarily on its ability to bind directly to the 40S subunit. Binding of SRP9/14 to 40S and Alu RNA is mutually exclusive indicating that the protein alone is bound to 40S in SGs and that Alu RNA might competitively regulate 40S binding. Indeed, by changing the effective Alu RNA concentration in the cell or by expressing an Alu RNA binding-defective protein we were able to influence SG formation and disassembly. Our findings suggest a model in which SRP9/14 binding to 40S promotes SG formation whereas the increase in cytoplasmic Alu RNA following stress promotes disassembly of SGs by disengaging SRP9/14 from 40S.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

