Snapshots of pre-rRNA structural flexibility reveal eukaryotic 40S assembly dynamics at nucleotide resolution
- PMID: 25200078
- PMCID: PMC4231735
- DOI: 10.1093/nar/gku815
Snapshots of pre-rRNA structural flexibility reveal eukaryotic 40S assembly dynamics at nucleotide resolution
Abstract
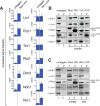
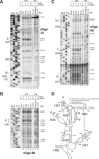
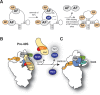
Ribosome assembly in eukaryotes involves the activity of hundreds of assembly factors that direct the hierarchical assembly of ribosomal proteins and numerous ribosomal RNA folding steps. However, detailed insights into the function of assembly factors and ribosomal RNA folding events are lacking. To address this, we have developed ChemModSeq, a method that combines structure probing, high-throughput sequencing and statistical modeling, to quantitatively measure RNA structural rearrangements during the assembly of macromolecular complexes. By applying ChemModSeq to purified 40S assembly intermediates we obtained nucleotide-resolution maps of ribosomal RNA flexibility revealing structurally distinct assembly intermediates and mechanistic insights into assembly dynamics not readily observed in cryo-electron microscopy reconstructions. We show that RNA restructuring events coincide with the release of assembly factors and predict that completion of the head domain is required before the Rio1 kinase enters the assembly pathway. Collectively, our results suggest that 40S assembly factors regulate the timely incorporation of ribosomal proteins by delaying specific folding steps in the 3' major domain of the 20S pre-ribosomal RNA.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Lafontaine D., Delcour J., Glasser A.L., Desgres J., Vandenhaute J. The DIM1 gene responsible for the conserved m6(2)Am6(2)A dimethylation in the 3′-terminal loop of 18 S rRNA is essential in yeast. J. Mol. Biol. 1994;241:492–497. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

