Distinct tmRNA sequence elements facilitate RNase R engagement on rescued ribosomes for selective nonstop mRNA decay
- PMID: 25200086
- PMCID: PMC4176180
- DOI: 10.1093/nar/gku802
Distinct tmRNA sequence elements facilitate RNase R engagement on rescued ribosomes for selective nonstop mRNA decay
Abstract
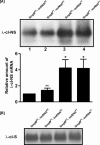
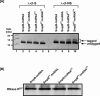
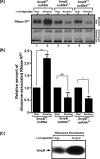
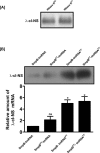
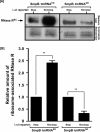
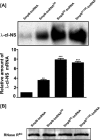
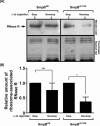
trans-Translation, orchestrated by SmpB and tmRNA, is the principal eubacterial pathway for resolving stalled translation complexes. RNase R, the leading nonstop mRNA surveillance factor, is recruited to stalled ribosomes in a trans-translation dependent process. To elucidate the contributions of SmpB and tmRNA to RNase R recruitment, we evaluated Escherichia coli-Francisella tularensis chimeric variants of tmRNA and SmpB. This evaluation showed that while the hybrid tmRNA supported nascent polypeptide tagging and ribosome rescue, it suffered defects in facilitating RNase R recruitment to stalled ribosomes. To gain further insights, we used established tmRNA and SmpB variants that impact distinct stages of the trans-translation process. Analysis of select tmRNA variants revealed that the sequence composition and positioning of the ultimate and penultimate codons of the tmRNA ORF play a crucial role in recruiting RNase R to rescued ribosomes. Evaluation of defined SmpB C-terminal tail variants highlighted the importance of establishing the tmRNA reading frame, and provided valuable clues into the timing of RNase R recruitment to rescued ribosomes. Taken together, these studies demonstrate that productive RNase R-ribosomes engagement requires active trans-translation, and suggest that RNase R captures the emerging nonstop mRNA at an early stage after establishment of the tmRNA ORF as the surrogate mRNA template.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
References
-
- Karzai A.W., Roche E.D., Sauer R.T. The SsrA-SmpB system for protein tagging, directed degradation and ribosome rescue. Nat. Struct. Biol. 2000;7:449–455. - PubMed
-
- Withey J.H., Friedman D.I. A salvage pathway for protein structures: tmRNA and trans-translation. Annu. Rev. Microbiol. 2003;57:101–123. - PubMed
-
- Dulebohn D., Choy J., Sundermeier T., Okan N., Karzai A.W. Trans-translation: the tmRNA-mediated surveillance mechanism for ribosome rescue, directed protein degradation, and nonstop mRNA decay. Biochemistry. 2007;46:4681–4693. - PubMed
-
- Barends S., Kraal B., van Wezel G.P. The tmRNA-tagging mechanism and the control of gene expression: a review. Wiley Interdiscip. Rev. RNA. 2011;2:233–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

