Characterization of diffuse fibrosis in the failing human heart via diffusion tensor imaging and quantitative histological validation
- PMID: 25200106
- PMCID: PMC4215542
- DOI: 10.1002/nbm.3200
Characterization of diffuse fibrosis in the failing human heart via diffusion tensor imaging and quantitative histological validation
Abstract
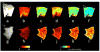
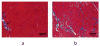
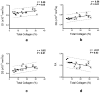
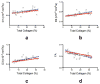
Non-invasive imaging techniques are highly desirable as an alternative to conventional biopsy for the characterization of the remodeling of tissues associated with disease progression, including end-stage heart failure. Cardiac diffusion tensor imaging (DTI) has become an established method for the characterization of myocardial microstructure. However, the relationships between diffuse myocardial fibrosis, which is a key biomarker for staging and treatment planning of the failing heart, and measured DTI parameters have yet to be investigated systematically. In this study, DTI was performed on left ventricular specimens collected from patients with chronic end-stage heart failure as a result of idiopathic dilated cardiomyopathy (n = 14) and from normal donors (n = 5). Scalar DTI parameters, including fractional anisotropy (FA) and mean (MD), primary (D1 ), secondary (D2 ) and tertiary (D3 ) diffusivities, were correlated with collagen content measured by digital microscopy. Compared with hearts from normal subjects, the FA in failing hearts decreased by 22%, whereas the MD, D2 and D3 increased by 12%, 14% and 24%, respectively (P < 0.01). No significant change was detected for D1 between the two groups. Furthermore, significant correlation was observed between the DTI scalar indices and quantitative histological measurements of collagen (i.e. fibrosis). Pearson's correlation coefficients (r) between collagen content and FA, MD, D2 and D3 were -0.51, 0.59, 0.56 and 0.62 (P < 0.05), respectively. The correlation between D1 and collagen content was not significant (r = 0.46, P = 0.05). Computational modeling analysis indicated that the behaviors of the DTI parameters as a function of the degree of fibrosis were well explained by compartmental exchange between myocardial and collagenous tissues. Combined, these findings suggest that scalar DTI parameters can be used as metrics for the non-invasive assessment of diffuse fibrosis in failing hearts.
Keywords: DTI; diffuse fibrosis; fractional anisotropy; histological correlation; idiopathic dilated cardiomyopathy; mean diffusivity; non-ischemic heart failure; principal diffusivities.
Copyright © 2014 John Wiley & Sons, Ltd.
Figures
References
-
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit Ja, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American HeartAssociation. Circulation. 2011;123(4):e18–e209. - PMC - PubMed
-
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJV, Ponikowski P, Poole-Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, Vahanian A, Camm J, De Caterina R, Dean V, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Auricchio A, Bax J, Böhm M, Corrà U, Della Bella P, Elliott PM, Follath F, Gheorghiade M, Hasin Y, Hernborg A, Jaarsma T, Komajda M, Kornowski R, Piepoli M, Prendergast B, Tavazzi L, Vachiery JL, Verheugt FWa, Zannad F. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J. 2008;29(19):2388–442. - PubMed
-
- Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111(21):2837–49. - PubMed
-
- Tamaki S, Mano T, Sakata Y, Ohtani T, Takeda Y, Kamimura D, Omori Y, Tsukamoto Y, Ikeya Y, Kawai M, Kumanogoh A, Hagihara K, Ishii R, Higashimori M, Kaneko M, Hasuwa H, Miwa T, Yamamoto K, Komuro I. Interleukin-16 promotes cardiac fibrosis and myocardial stiffening in heart failure with preserved ejection fraction. PLoS One. 2013;8(7):e68893. - PMC - PubMed
-
- Ten Tusscher KHWJ, Panfilov AV. Influence of diffuse fibrosis on wave propagation in human ventricular tissue. Europace. 2007;(9) Suppl 6:vi38–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

