Pharmacokinetic and pharmacodynamic profiles of canagliflozin in Japanese patients with type 2 diabetes mellitus and moderate renal impairment
- PMID: 25200141
- PMCID: PMC4168221
- DOI: 10.1007/s40261-014-0226-x
Pharmacokinetic and pharmacodynamic profiles of canagliflozin in Japanese patients with type 2 diabetes mellitus and moderate renal impairment
Abstract
Background and objectives: This study examined the effects of moderate renal impairment on the pharmacokinetics and pharmacodynamics of canagliflozin in Japanese patients with type 2 diabetes mellitus.
Methods: Japanese patients with stable type 2 diabetes (12 with moderate renal impairment and 12 with normal renal function or mild renal impairment) were eligible. This was an open-label, randomized, two-way crossover, two-sequence, single-dose study performed at a single center in Japan. The subjects were hospitalized for the pharmacodynamic/pharmacokinetic evaluations. Twenty-four patients received a single dose each of canagliflozin 100 and 200 mg before breakfast in a crossover manner with a 14-day washout between doses. The main outcome measures were pharmacokinetics of canagliflozin and its main metabolites (M5 and M7) in plasma and urine, and change from baseline in 24-h urinary glucose excretion (ΔUGE24 h).
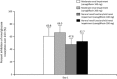
Results: There was no significant effect of moderate renal impairment on the maximum canagliflozin concentration. The ratios of least square means (90 % confidence intervals [CIs]) of moderate renal impairment relative to normal renal function or mild renal impairment were 0.982 (0.821-1.173) and 0.989 (0.827-1.182) for the 100 and 200 mg doses, respectively. The canagliflozin area under the plasma concentration-time curve was greater in those with moderate renal impairment than in those without, after both canagliflozin doses (ratio of least square means [90 % CI] 1.258 [1.061-1.490] and 1.216 [1.026-1.441]). ΔUGE24 h increased after administration of both doses, but in patients with moderate renal impairment, the increase was approximately 70 % of that in patients with normal renal function or mild renal impairment. The incidence of adverse events was low and no patient developed hypoglycemia.
Conclusion: The pharmacokinetics of canagliflozin are affected by renal function, with slight decreases in renal clearance observed. No effect of renal impairment on the maximum concentration was observed. Renal impairment reduced the ability of canagliflozin to promote urinary glucose excretion.
Figures



References
-
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

