Connexin40 abnormalities and atrial fibrillation in the human heart
- PMID: 25200600
- PMCID: PMC4250516
- DOI: 10.1016/j.yjmcc.2014.08.021
Connexin40 abnormalities and atrial fibrillation in the human heart
Abstract
Normal atrial conduction requires similar abundances and homogeneous/overlapping distributions of two connexins (Cx40 and Cx43). The remodeling of myocyte connections and altered electrical conduction associated with atrial fibrillation (AF) likely involves perturbations of these connexins. We conducted a comprehensive series of experiments to examine the abundances and distributions of Cx40 and Cx43 in the atria of AF patients. Atrial appendage tissues were obtained from patients with lone AF (paroxysmal or chronic) or normal controls. Connexins were localized by double label immunofluorescence confocal microscopy, and their overlap was quantified. Connexin proteins and mRNAs were quantified by immunoblotting and qRT-PCR. PCR amplified genomic DNA was sequenced to screen for connexin gene mutations or polymorphisms. Immunoblotting showed reductions of Cx40 protein (to 77% or 49% of control values in samples from patients with paroxysmal and chronic AF, respectively), but no significant changes of Cx43 protein levels in samples from AF patients. The extent of Cx43 immunostaining and its distribution relative to N-cadherin were preserved in the AF patient samples. Although there was variability of Cx40 staining among paroxysmal AF patients, all had some fields with substantial Cx40 heterogeneity and reduced overlap with Cx43. Cx40 immunostaining was severely reduced in all chronic AF patients. qRT-PCR showed no change in Cx43 mRNA levels, but reductions in total Cx40 mRNA (to <50%) and Cx40 transcripts A (to ~50%) and B (to <25%) as compared to controls. No Cx40 coding region mutations were identified. The frequency of promoter polymorphisms did not differ between AF patient samples and controls. Our data suggest that reduced Cx40 levels and heterogeneity of its distribution (relative to Cx43) are common in AF. Multiple mechanisms likely lead to reductions of functional Cx40 in atrial gap junctions and contribute to the pathogenesis of AF in different patients.
Keywords: Arrhythmia; Atrial fibrillation; Connexin; Connexin40; Gap junction.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

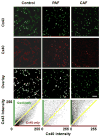


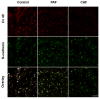
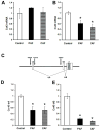
References
-
- Allessie MA, Boyden PA, Camm AJ, kleber AG, Lab MJ, Legato MJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103:769–77. - PubMed
-
- Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–74. - PubMed
-
- Spach MS, Starmer CF. Altering the topology of gap junctions: a major therapeutic target for atrial fibrillation. Cardiovasc Res. 1995;30:337–44. - PubMed
-
- Saffitz JE, Kanter HL, Green KG, Tolley TK, Beyer EC. Tissue-specific determinants of anisotropic conduction velocity in canine atrial and ventricular myocardium. Circ Res. 1994;74:1065–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

