Human dermal CD14⁺ cells are a transient population of monocyte-derived macrophages
- PMID: 25200712
- PMCID: PMC4175180
- DOI: 10.1016/j.immuni.2014.08.006
Human dermal CD14⁺ cells are a transient population of monocyte-derived macrophages
Erratum in
- Immunity. 2015 Feb 17;42(2):391. Saavedra, Diego Miranda [corrected to Miranda-Saavedra, Diego]
-
Population of Monocyte-Derived Macrophages.Immunity. 2015 Feb 17;42(2):391. doi: 10.1016/j.immuni.2015.01.018. Epub 2015 Feb 17. Immunity. 2015. PMID: 28843277 Free PMC article. No abstract available.
Abstract
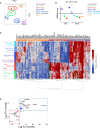
Dendritic cells (DCs), monocytes, and macrophages are leukocytes with critical roles in immunity and tolerance. The DC network is evolutionarily conserved; the homologs of human tissue CD141(hi)XCR1⁺ CLEC9A⁺ DCs and CD1c⁺ DCs are murine CD103⁺ DCs and CD64⁻ CD11b⁺ DCs. In addition, human tissues also contain CD14⁺ cells, currently designated as DCs, with an as-yet unknown murine counterpart. Here we have demonstrated that human dermal CD14⁺ cells are a tissue-resident population of monocyte-derived macrophages with a short half-life of <6 days. The decline and reconstitution kinetics of human blood CD14⁺ monocytes and dermal CD14⁺ cells in vivo supported their precursor-progeny relationship. The murine homologs of human dermal CD14⁺ cells are CD11b⁺ CD64⁺ monocyte-derived macrophages. Human and mouse monocytes and macrophages were defined by highly conserved gene transcripts, which were distinct from DCs. The demonstration of monocyte-derived macrophages in the steady state in human tissue supports a conserved organization of human and mouse mononuclear phagocyte system.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




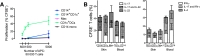

References
-
- Angel C.E., George E., Brooks A.E., Ostrovsky L.L., Brown T.L., Dunbar P.R. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J. Immunol. 2006;176:5730–5734. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

