Novel mechanism of impaired function of organic anion-transporting polypeptide 1B3 in human hepatocytes: post-translational regulation of OATP1B3 by protein kinase C activation
- PMID: 25200870
- PMCID: PMC4201128
- DOI: 10.1124/dmd.114.056945
Novel mechanism of impaired function of organic anion-transporting polypeptide 1B3 in human hepatocytes: post-translational regulation of OATP1B3 by protein kinase C activation
Abstract
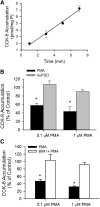
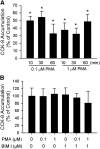
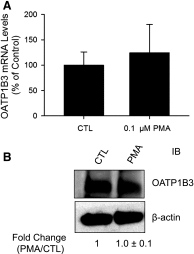
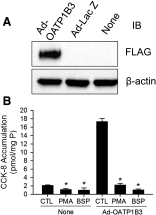
The organic anion-transporting polypeptide (OATP) 1B3 is a membrane transport protein that mediates hepatic uptake of many drugs and endogenous compounds. Currently, determination of OATP-mediated drug-drug interactions in vitro is focused primarily on direct substrate inhibition. Indirect inhibition of OATP1B3 activity is under-appreciated. OATP1B3 has putative protein kinase C (PKC) phosphorylation sites. Studies were designed to determine the effect of PKC activation on OATP1B3-mediated transport in human hepatocytes using cholecystokinin-8 (CCK-8), a specific OATP1B3 substrate, as the probe. A PKC activator, phorbol-12-myristate-13-acetate (PMA), did not directly inhibit [(3)H]CCK-8 accumulation in human sandwich-cultured hepatocytes (SCH). However, pretreatment with PMA for as little as 10 minutes rapidly decreased [(3)H]CCK-8 accumulation. Treatment with a PKC inhibitor bisindolylmaleimide (BIM) I prior to PMA treatment blocked the inhibitory effect of PMA, indicating PKC activation is essential for downregulating OATP1B3 activity. PMA pretreatment did not affect OATP1B3 mRNA or total protein levels. To determine the mechanism(s) underlying the indirect inhibition of OATP1B3 activity upon PKC activation, adenoviral vectors expressing FLAG-Myc-tagged OATP1B3 (Ad-OATP1B3) were transduced into human hepatocytes; surface expression and phosphorylation of OATP1B3 were determined by biotinylation and by an anti-phosphor-Ser/Thr/Tyr antibody, respectively. PMA pretreatment markedly increased OATP1B3 phosphorylation without affecting surface or total OATP1B3 protein levels. In conclusion, PKC activation rapidly decreases OATP1B3 transport activity by post-translational regulation of OATP1B3. These studies elucidate a novel indirect inhibitory mechanism affecting hepatic uptake mediated by OATP1B3, and provide new insights into predicting OATP-mediated drug interactions between OATP substrates and kinase modulator drugs/endogenous compounds.
Copyright © 2014 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


Similar articles
-
Regulation of Organic Anion Transporting Polypeptides (OATP) 1B1- and OATP1B3-Mediated Transport: An Updated Review in the Context of OATP-Mediated Drug-Drug Interactions.Int J Mol Sci. 2018 Mar 14;19(3):855. doi: 10.3390/ijms19030855. Int J Mol Sci. 2018. PMID: 29538325 Free PMC article. Review.
-
Development of a Rat Sandwich-Cultured Hepatocytes Model Expressing Functional Human Organic Anion Transporting Polypeptide (OATP) 1B3: A Potential Screening Tool for Liver-Targeting Compounds.J Pharm Pharm Sci. 2021;24:475-483. doi: 10.18433/jpps31818. J Pharm Pharm Sci. 2021. PMID: 34516949 Free PMC article.
-
Treatment with proteasome inhibitor bortezomib decreases organic anion transporting polypeptide (OATP) 1B3-mediated transport in a substrate-dependent manner.PLoS One. 2017 Nov 6;12(11):e0186924. doi: 10.1371/journal.pone.0186924. eCollection 2017. PLoS One. 2017. PMID: 29107984 Free PMC article.
-
Pre-incubation with OATP1B1 and OATP1B3 inhibitors potentiates inhibitory effects in physiologically relevant sandwich-cultured primary human hepatocytes.Eur J Pharm Sci. 2021 Oct 1;165:105951. doi: 10.1016/j.ejps.2021.105951. Epub 2021 Jul 24. Eur J Pharm Sci. 2021. PMID: 34311070 Free PMC article.
-
[Advances in the study of organic anion transporting polypeptide 1B3].Yao Xue Xue Bao. 2011 Nov;46(11):1279-85. Yao Xue Xue Bao. 2011. PMID: 22260016 Review. Chinese.
Cited by
-
Loops and layers of post-translational modifications of drug transporters.Adv Drug Deliv Rev. 2017 Jul 1;116:37-44. doi: 10.1016/j.addr.2016.05.003. Epub 2016 May 9. Adv Drug Deliv Rev. 2017. PMID: 27174152 Free PMC article. Review.
-
Characterization of Plasma Membrane Localization and Phosphorylation Status of Organic Anion Transporting Polypeptide (OATP) 1B1 c.521 T>C Nonsynonymous Single-Nucleotide Polymorphism.Pharm Res. 2019 May 15;36(7):101. doi: 10.1007/s11095-019-2634-3. Pharm Res. 2019. PMID: 31093828 Free PMC article.
-
Assessing Trans-Inhibition of OATP1B1 and OATP1B3 by Calcineurin and/or PPIase Inhibitors and Global Identification of OATP1B1/3-Associated Proteins.Pharmaceutics. 2023 Dec 31;16(1):63. doi: 10.3390/pharmaceutics16010063. Pharmaceutics. 2023. PMID: 38258074 Free PMC article.
-
Regulation of Organic Anion Transporting Polypeptides (OATP) 1B1- and OATP1B3-Mediated Transport: An Updated Review in the Context of OATP-Mediated Drug-Drug Interactions.Int J Mol Sci. 2018 Mar 14;19(3):855. doi: 10.3390/ijms19030855. Int J Mol Sci. 2018. PMID: 29538325 Free PMC article. Review.
-
Downregulation of Organic Anion Transporting Polypeptide (OATP) 1B1 Transport Function by Lysosomotropic Drug Chloroquine: Implication in OATP-Mediated Drug-Drug Interactions.Mol Pharm. 2016 Mar 7;13(3):839-51. doi: 10.1021/acs.molpharmaceut.5b00763. Epub 2016 Feb 1. Mol Pharm. 2016. PMID: 26750564 Free PMC article.
References
-
- Annaert P, Ye ZW, Stieger B, Augustijns P. (2010) Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica 40:163–176 - PubMed
-
- Conradt M, Stoffel W. (1997) Inhibition of the high-affinity brain glutamate transporter GLAST-1 via direct phosphorylation. J Neurochem 68:1244–1251 - PubMed
-
- Cui Y, König J, Leier I, Buchholz U, Keppler D. (2001) Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem 276:9626–9630 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

